Organic dye-stuff with multi-heterocycle derivant as conjugation unit and dye sensitized solar battery produced with the same
A technology of organic dyes and derivatives, applied in the field of dye-sensitized solar cells, can solve problems such as limiting practical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The synthetic route of dye I is as follows:
[0071]
[0072] The specific synthesis method is:
[0073] Compound c10g, compound d10.3g, Pd(PPh 3 ) 4 2.2 g, 250 mL of toluene were refluxed for 12 hours under the protection of argon, the solvent was removed, and the compound e was obtained by column chromatography. Dissolve compound e2.4g in 30mL DMF, protect with argon, cool to 0°C, add dropwise the solution of NBS in DMF, continue to complete the reaction after the addition, extract with chloroform, wash with water, dry over magnesium sulfate, remove the solvent and perform column chromatography Compound f. Take compound f1.3g, compound g0.72g, Pd(PPh 3 ) 4 0.23g, K 2 CO 3(2M) solution 6.9mL and THF 70mL were refluxed for 12h under the protection of argon, the solvent was removed, and the compound h was obtained by column chromatography. Compound h0.7g, POCl 3 0.2mL, 0.75mL of DMF and 10mL of dichloroethane were refluxed for 6h under the protection of argon...
Embodiment 2
[0076] The synthetic route of dye II is as follows:
[0077]
[0078] The specific synthesis method is:
[0079] Take compound f 1.3g, compound j 1.05g, Pd(PPh 3 ) 4 0.23g, K 2 CO 3 (2M) solution 6.9mL and THF 70mL were refluxed for 12h under the protection of argon, the solvent was removed, and compound k was obtained by column chromatography. Compound k 0.4g, POCl 3 0.1mL, DMF 0.4mL and dichloroethane 10mL were refluxed for 6h under the protection of argon, diluted with chloroform, washed with water, saturated sodium carbonate solution and saturated brine successively, dried over magnesium sulfate, and concentrated column chromatography to obtain compound 1. 0.2 g of compound 1, 0.1 g of cyanoacetic acid, 0.1 mL of piperidine, and 5 mL of chloroform were refluxed overnight under the protection of argon, the solvent was removed, and the dye II was obtained by column chromatography.
[0080] NMR data of dye II: 1 H NMR (400MHz, DMSO, δ H ): 0.88(t, 6H), 1.32(m, 8H)...
Embodiment 3
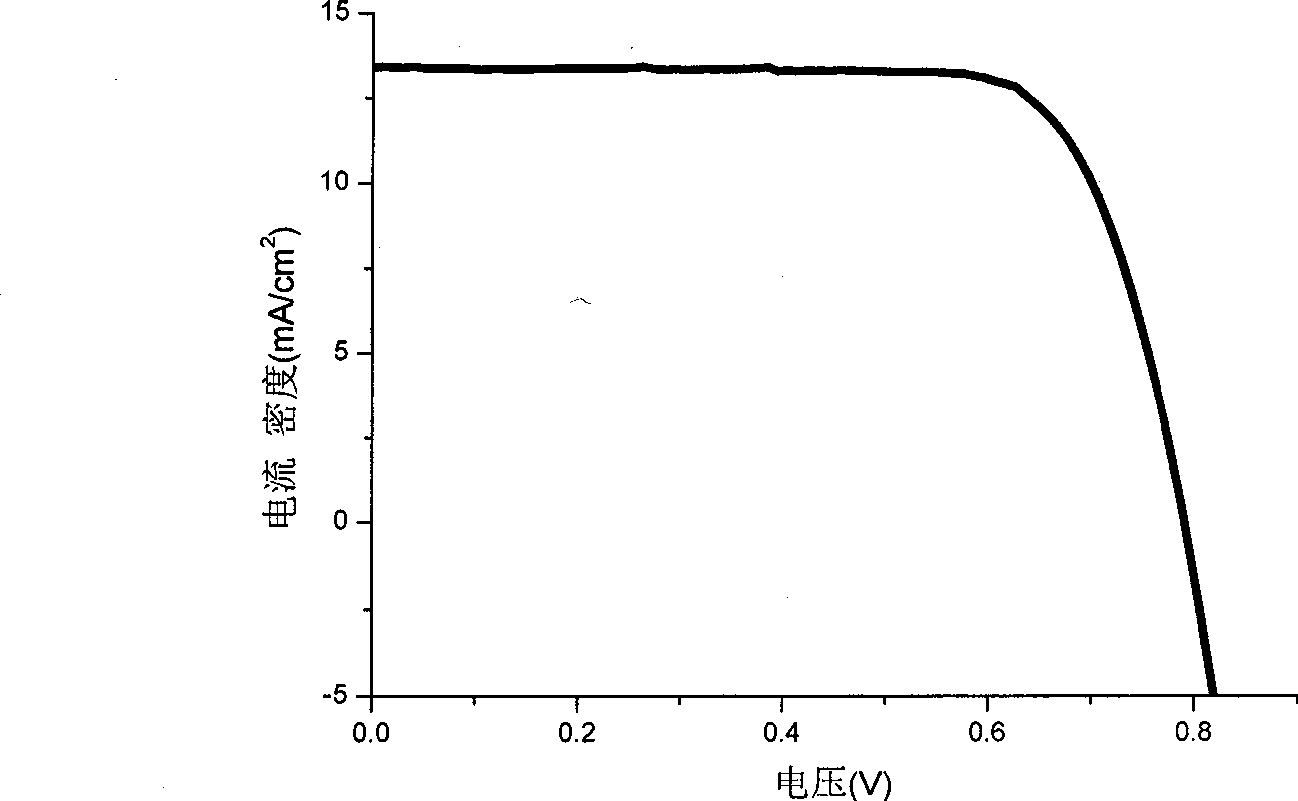
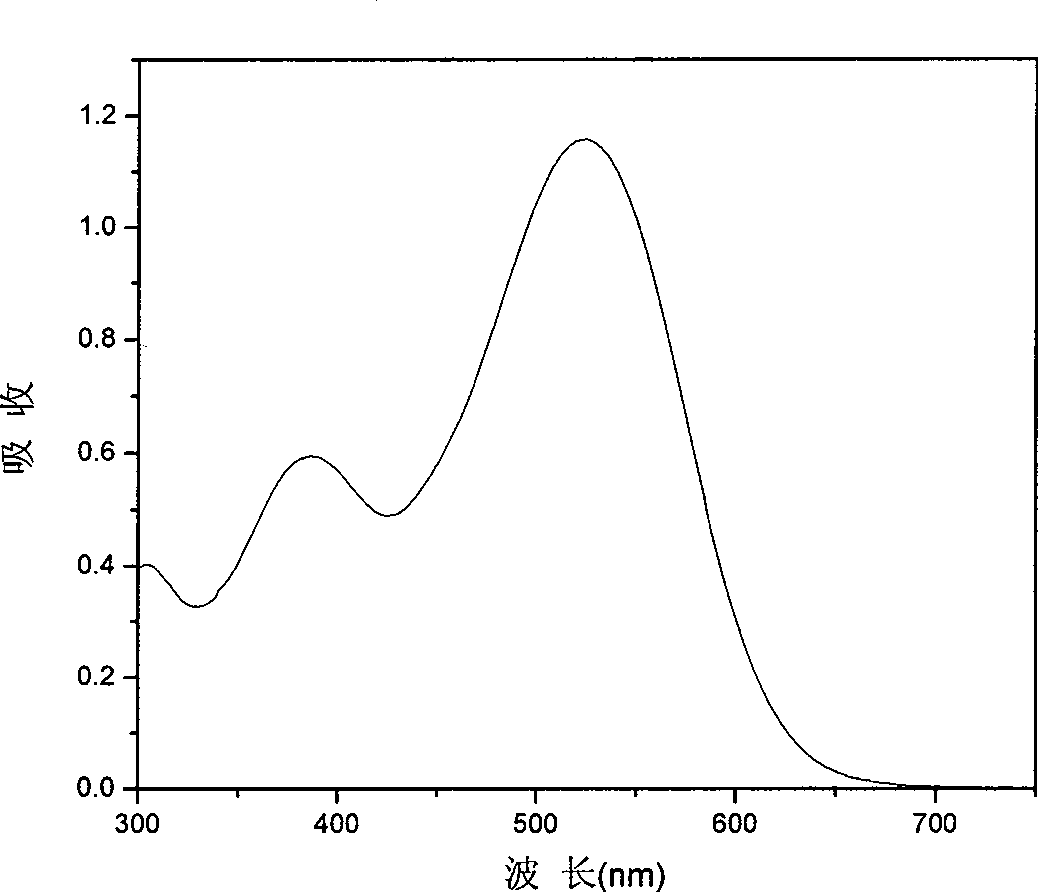
[0081] Example 3: Dye-sensitized solar cells prepared by organic dyes I
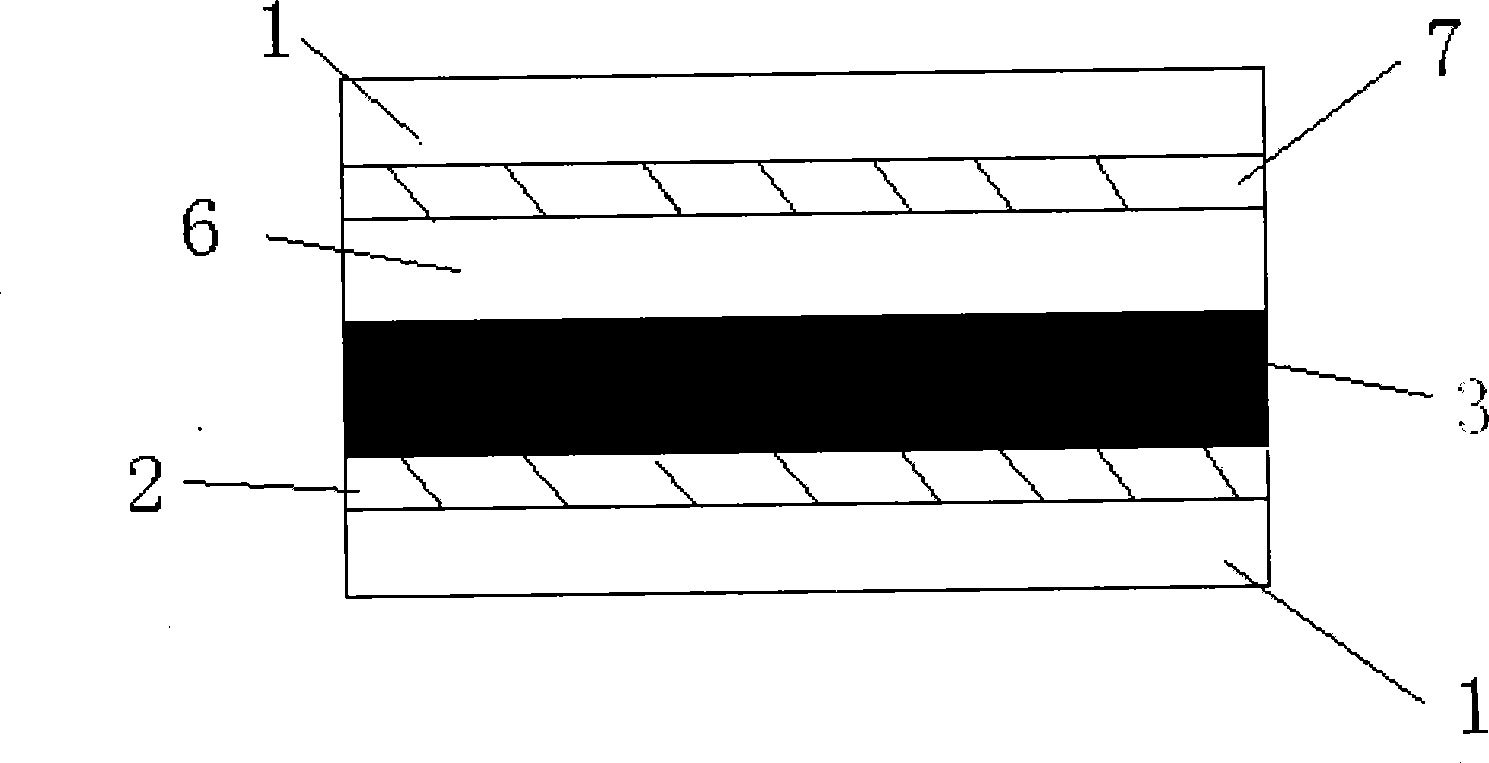
[0082] TiO with a particle size of 20nm 2 Colloid coating on fluorine-doped SnO 2 On conductive glass, nano-TiO is formed 2 The crystal film was baked at 400 °C for 12 hours to obtain TiO with a thickness of 7 μm 2 crystal film; the obtained TiO 2 Using the same method on the layer film, the particle size is 400nmTiO 2 , fired TiO with a thickness of 5 μm 2 Light scattering film; obtained TiO 2 Nanostructured bilayer membrane electrodes. Specific preparation of TiO 2 Nanocrystalline and TiO 2 For the method of nanostructured double-layer membrane electrode, see the article (Wang P. et al., Enhance the Performance of Dye-Sensitized SolarCells by Co-grafting Amphiphilic Sensitizer and Hexadecylmalonic Acidon TiO 2 Nanocrystals, J. Phys. Chem. B., 107, 2003, 14336).
[0083] Prepared TiO 2 The nanostructured double-layer membrane electrode was immersed in a chlorobenzene solution containing 300 μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Short circuit photocurrent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com