Polybrominated diphenyl ethylene dye sensitizing agent, synthesis and use thereof
A technology of polybrominated stilbenes and dye sensitizers, applied in the preparation of styrene-based dyes and organic compounds, and the preparation of amino compounds from amines, etc., can solve the problems of poor singlet oxygen quantum yield, etc. Achieve the effects of convenient and easy-to-obtain raw material sources, wide coverage, and high quantum yield of singlet oxygen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
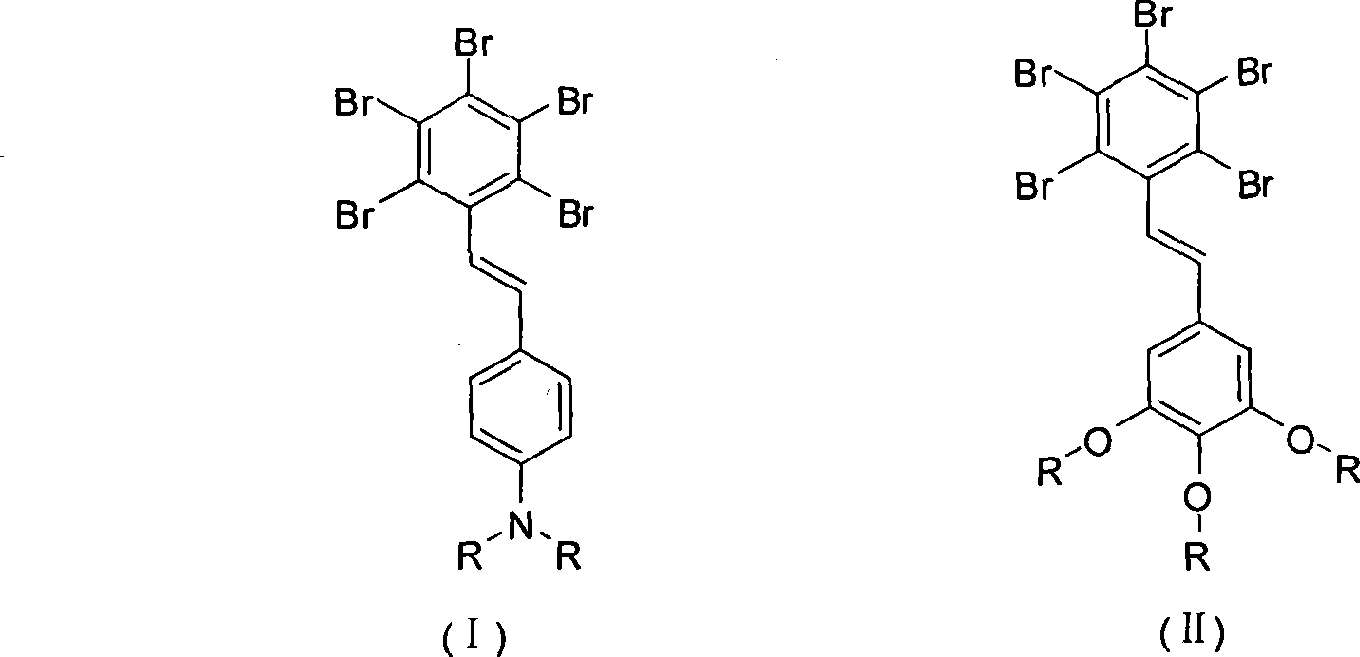
[0037] Synthesis of p-N, N-diphenyl-pentabromostilbene
[0038] The synthesis proceeds in three steps:
[0039] (1) Synthesis of brominated pentabromotoluene
[0040] 10 grams of pentabromotoluene and 3.7 grams of NBS (molar ratio 1:1) are mixed in a three-necked ground flask, and a condensing device is added, and in CCl 4 Reflux for 48 hours, filter, recrystallize the solid in benzene / cyclohexane, filter to obtain crystals, the yield is 75%, and set aside;
[0041] (2) Esterification of pentabromotoluene
[0042] Mix 5 grams of bromopentabromotoluene synthesized in the first step with 4.5 grams of triethyl phosphite (molar ratio 1:5) in a three-necked flask, stir at 130-160°C for 6 hours, and evaporate excess The triethyl phosphite, obtains white crystal, productive rate 95%, for subsequent use;
[0043] (3) Synthesis of p-N, N-diphenyl-pentabromostilbene
[0044] Pentabromotoluene and 1.74 gram p-N, N-diphenyl-benzaldehyde (molar ratio 1:1.2) after the 3.3 gram esterifi...
Embodiment 2
[0046] Synthesis of 3,4,5-trimethoxy-pentabromostilbene
[0047] The synthesis proceeds in three steps:
[0048] (1) Synthesis of brominated pentabromotoluene
[0049] Synthesis is by the first step in embodiment 1;
[0050] (2) Esterification of pentabromotoluene
[0051] Synthesis is by the second step in Example 1;
[0052] (3) Synthesis of 3,4,5-trimethoxy-pentabromostilbene
[0053] Pentabromotoluene and 0.83 gram 3,4,5-trimethoxyl-benzaldehyde (molar ratio 1:1.2) mixed with the 2.2 gram esterified pentabromotoluenes that the second step makes are added in the three-necked ground-necked flask, add Condenser, then dissolve with 200ml of dry tetrahydrofuran solvent, add 0.73 gram of sodium methylate, reflux for 12 hours, filter the precipitate after cooling, after the solvent in the filtrate is spin-dried in vacuum, adopt silica gel column separation to obtain the compound of the invention, the yield 38%.
Embodiment 3
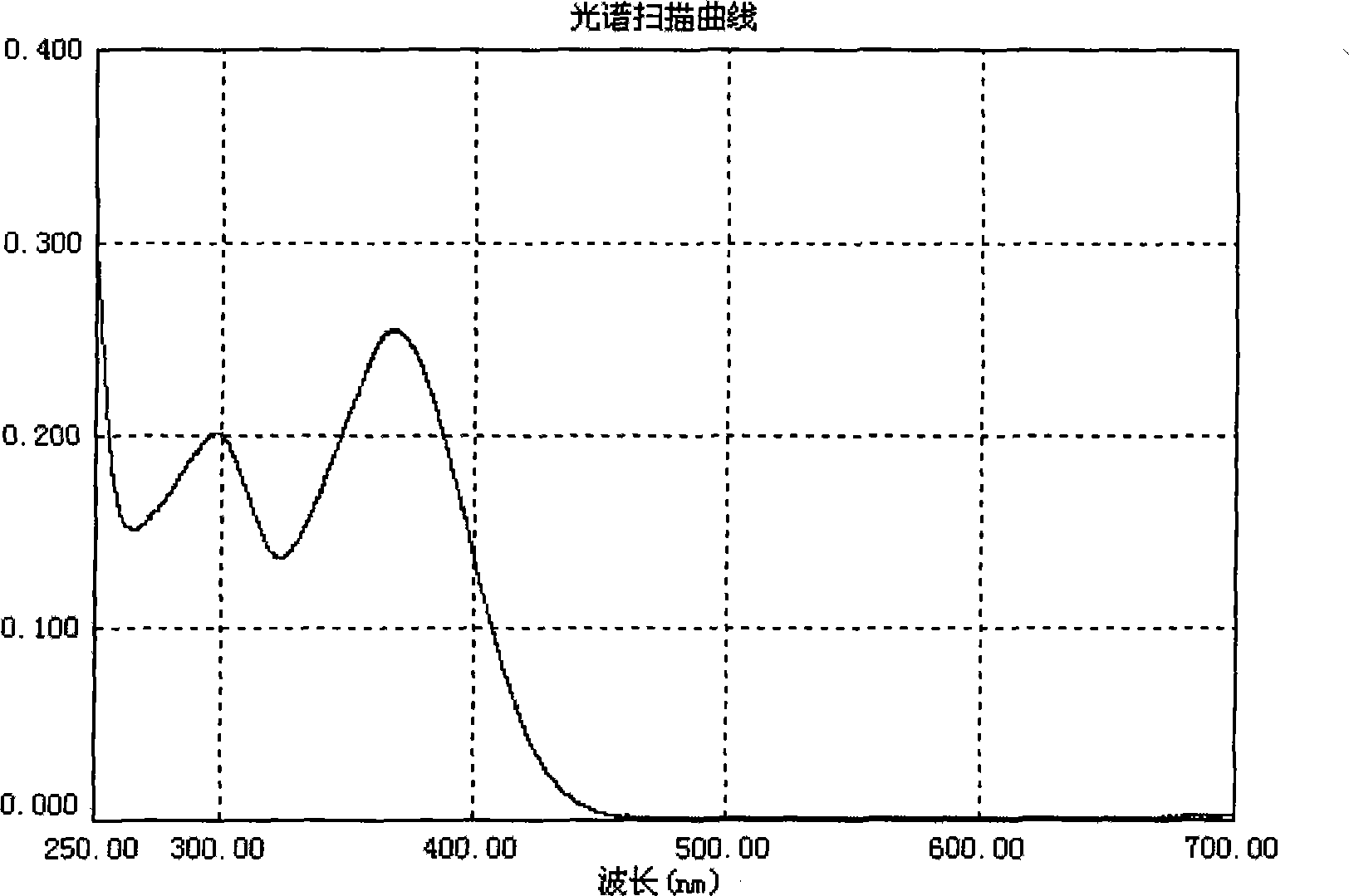
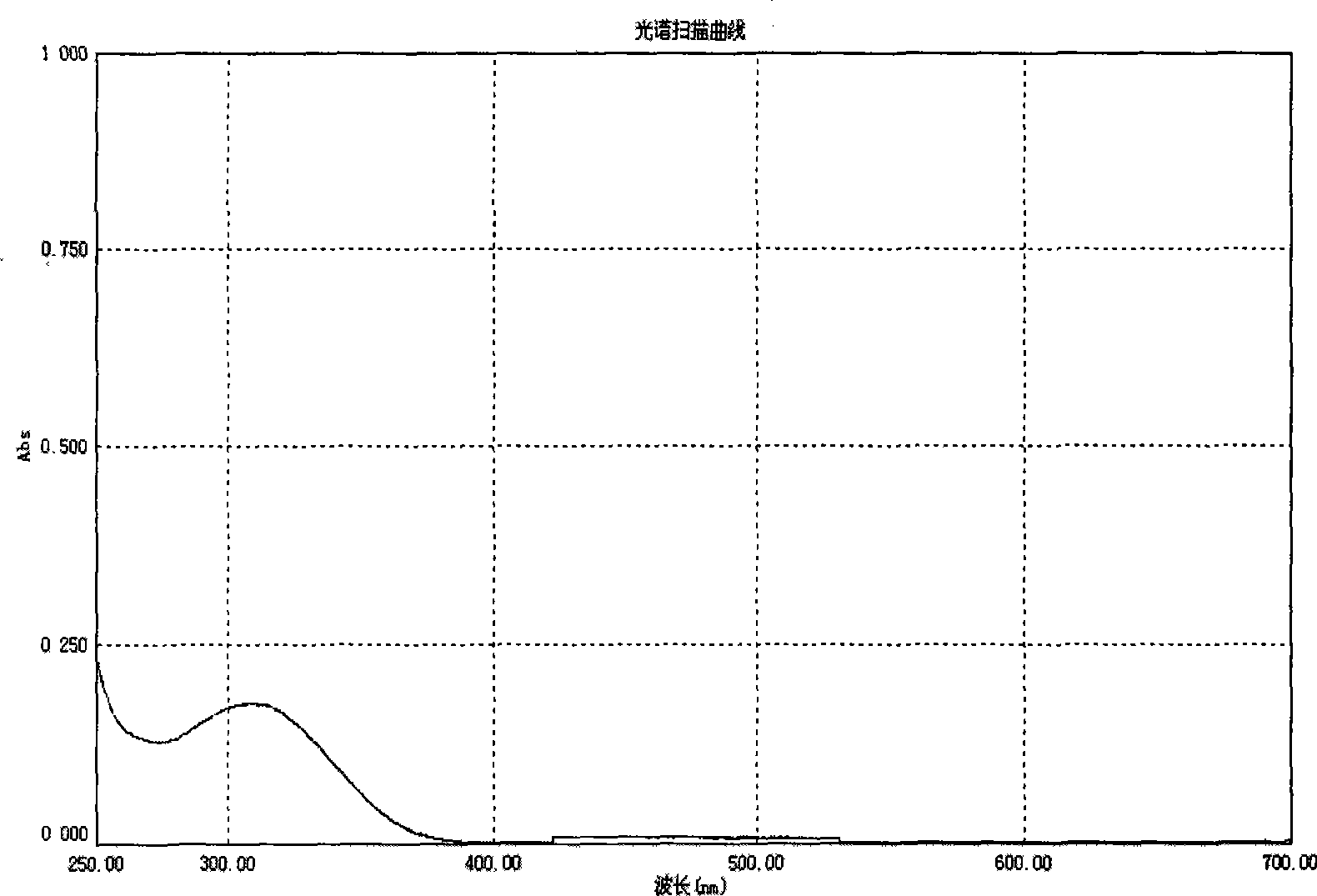
[0055] Will 1×10 -5 The p-N of mol / L, N-diphenyl-pentabromostilbene is dissolved in methylene chloride, and its ultraviolet-visible absorption spectrum is measured, as figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com