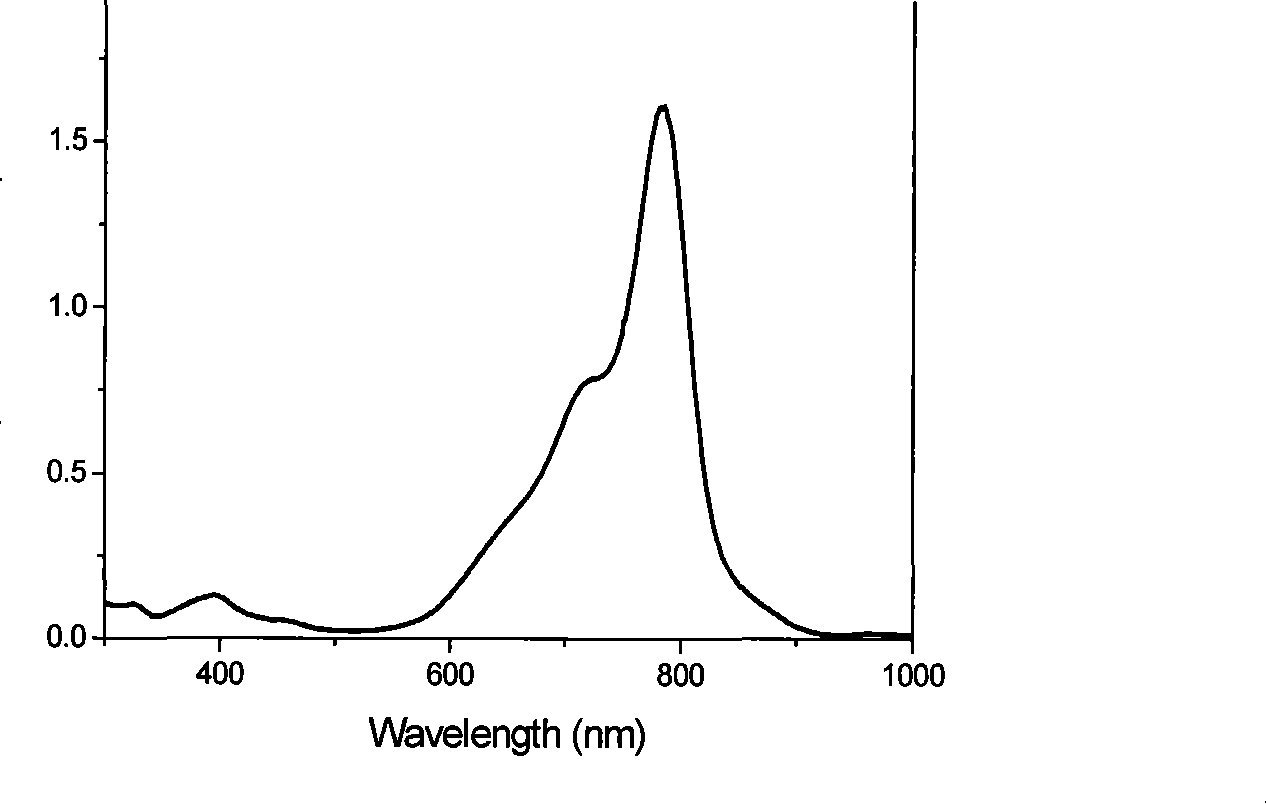
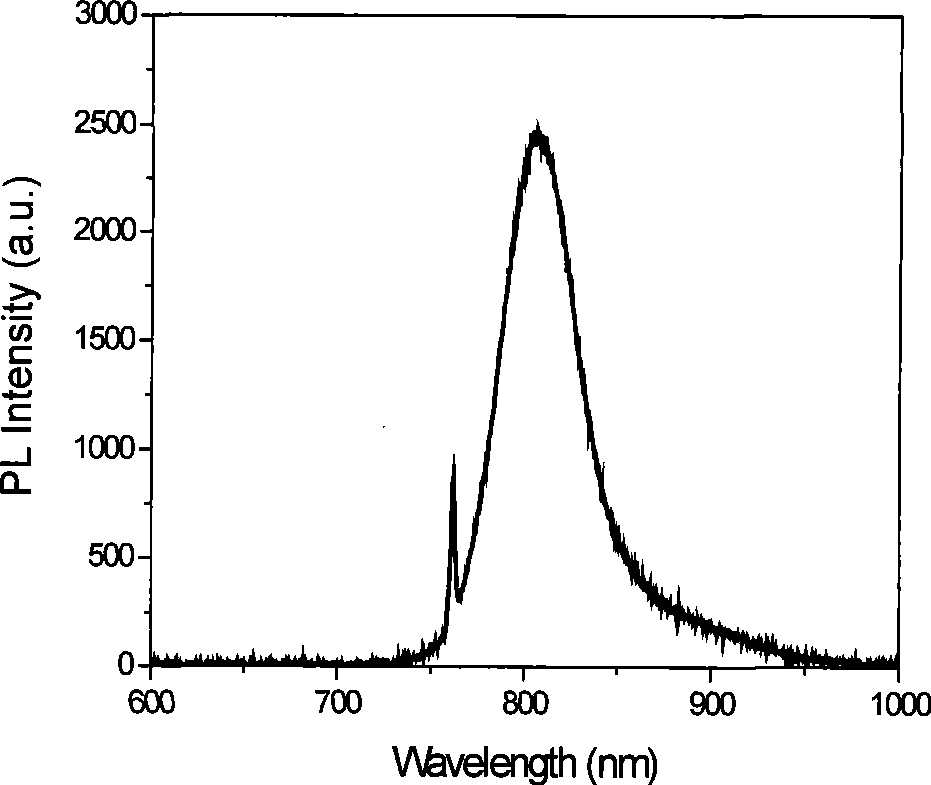
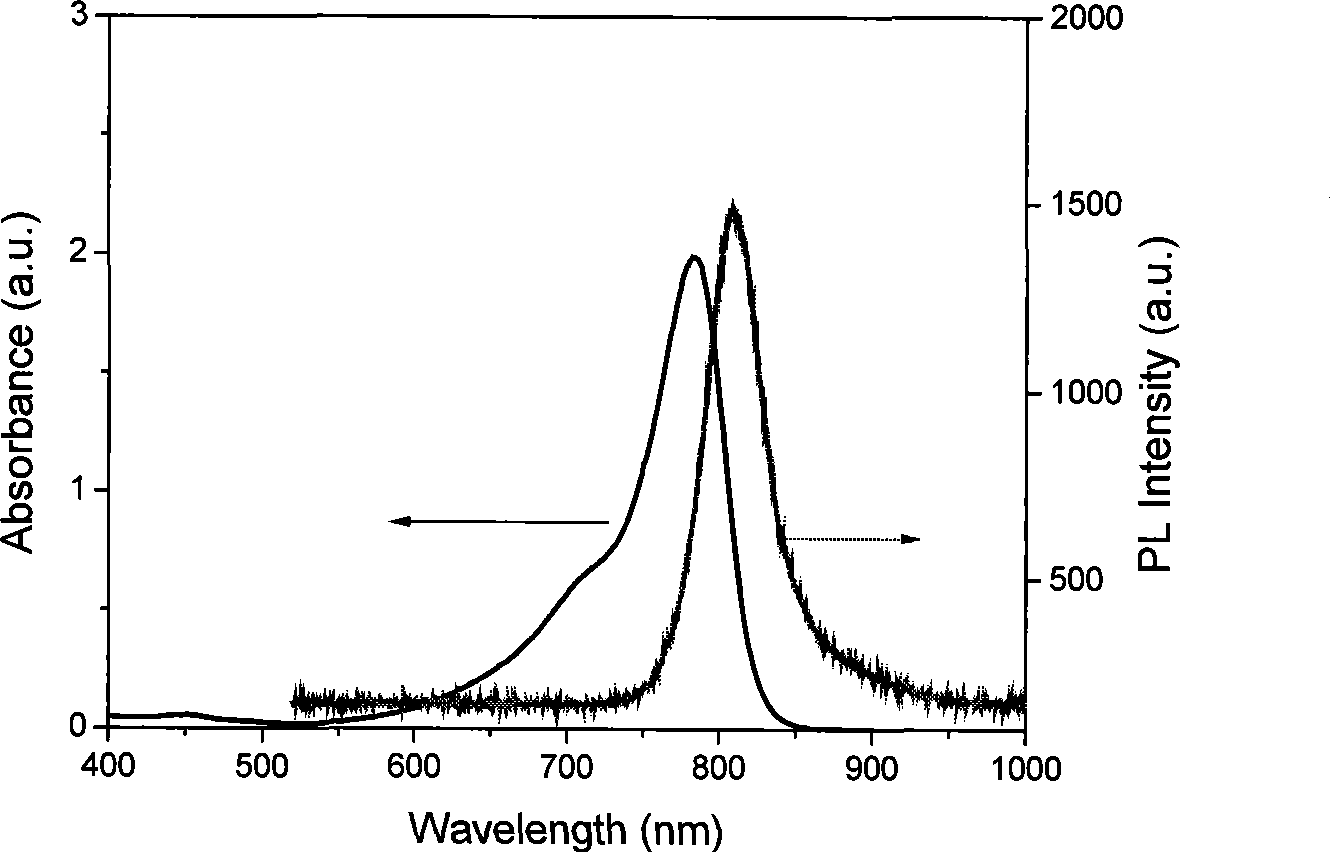
Near-infrared fluorescent molecular probe, synthesizing method and use thereof
A fluorescent molecular probe, near-infrared technology, applied in fluorescence/phosphorescence, chemical instruments and methods, biochemical equipment and methods, etc. The effect of increased compatibility, extended cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Preparation of ICG-Der-01
[0057] 1. Dissolve 4g of 1,1,2-trimethyl[1H]-benzindole and 6g of 3-bromopropionic acid in 30mL of 1,2-dichlorobenzene solution, and keep stirring at 130°C Heat for 16 hours. After the reaction solution was cooled to room temperature, dichloromethane was added to the reaction system to precipitate the product, and the product was washed three times with dichloromethane to remove unreacted substances, and finally the relatively pure product 1,1,2-trimethyl Base [1H]-benzoindole-3-propionic acid (dark purple).
[0058] 2. Dissolve 4g of 1,1,2-trimethyl[1H]-benzindole and 8g of iodocaproic acid in 30mL of acetonitrile, heat to boiling under constant stirring, and reflux for 4 days. After the reaction is finished, cool to room temperature, add dichloromethane to the reaction system to precipitate the product, and wash the product with dichloromethane three times to remove unreacted substances, and finally obtain the relatively pure product 1,1,...
Embodiment 2
[0061] Preparation of ICG-Der-02
[0062] 1. Add 30mL of glacial acetic acid to a mixture of p-hydrazinobenzenesulfonic acid (6g), methyl isopropyl ketone (7mL) and sodium acetate (2g). The obtained brown suspension was heated to boiling and refluxed for 16 hours. Then filter while hot with a sintered glass filter to remove unreacted suspended matter. After the filtrate was cooled to room temperature, the product was precipitated with dichloromethane, and the product was brown (2,2,3-trimethyl[3H]-indole-5-sulfonic acid).
[0063] 2. Take 50 mL o-dichlorobenzene and add to 2,2,3-trimethyl[3H]-indole-5-sulfonic acid (15 g) and 1,3-propane sultone (4 mL). The mixture was heated to 130°C and held for 15 hours. The resulting solid was triturated with dichloromethane to finally give the product 2,2,3-trimethyl-5-sulfonic acid-1-(3-sulfonic acid-propyl)-[3H]-indole.
[0064] 3. Add 20mL of absolute ethanol to 2,2,3-trimethyl-5-sulfonic acid-1-(3-sulfonic acid-propyl)-[3H]-indole...
Embodiment 3
[0067] Preparation of Folic Acid-Coupled ICG-Der-01 Near-infrared Fluorescent Probe Complex
[0068] Weigh 18 mg of folic acid (Folic acid) and dissolve it in 5 mL of anhydrous dimethylsulfoxide (DMSO), add 15 mg of 1-ethyl-3 (3-dimethylpropylamine) carbodiimide and 7 mg of N-hydroxysuccinyl Imine (EDCI / NHS) was activated for 2 hours, and the activation solution was added dropwise to an anhydrous dimethyl sulfoxide (DMSO) solution containing 200 mg of bisaminopolyethylene glycol (PEG-bis-amine, molecular weight 4,000), avoiding React at room temperature for 8 hours; Folate-PEG was obtained by separation and purification on a silica gel column. Take 2 mg of ICG-der-01 near-infrared dye containing carboxyl functional groups, dissolve it in 1 mL of anhydrous DMSO, add the catalyst EDCI / NHS to activate for 2 hours, and then add the activated solution dropwise to polyethylene glycol (Folate- PEG) in pH 9.4 carbonate buffer and reacted in the dark for 12 hours. The reaction soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com