Vascular substrate without cell in vascular tissue and preparation method thereof
A decellularization and vascular technology, applied in the field of vascular matrix and its preparation, can solve the problems of graft failure, vascular intolerance to pressure conditions, unfavorable recipient cell infiltration and growth, etc., and achieve the effect of low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Preparation of decellularized vascular matrix
[0047] 1. Experimental materials:
[0048] 1) Blood vessel: the iliac artery of a freshly slaughtered pig taken from a nearby slaughterhouse, with a diameter of about 4-5 mm and a length of about 5 cm.
[0049] 2) Reagents: 1% Triton X-100 (Sigma); 0.1% Ammonium Hydroxide (Sigma); PBS (GIBCO); 0.25% Trypsin-0.02% EDTA (Sigma); 10% Fetal Bovine Serum (FBS, GIBCO) ; Low sugar-DMEM medium (LG-DMEM, GIBCO);
[0050] 3) Instruments: TS-100 decolorization shaker (Beijing Binda Yingchuang Technology Co., Ltd.); DK-8D constant temperature water tank (Shanghai Jinghong Experimental Equipment Co., Ltd.); LP-400 spectrophotometer (France); S-4800 Scanning electron microscope (HITACHI, Japan); AutoPore IV 9510 mercury porosimeter (Micromeritics Instruments Inc.USA) Shimadzu AG-5000A (Japan);
[0051] 2. Experimental method:
[0052] The blood vessels were taken from the adjacent slaughterhouse to take the iliac artery of...
Embodiment 2
[0059] Example 2 Detection of decellularized vascular matrix
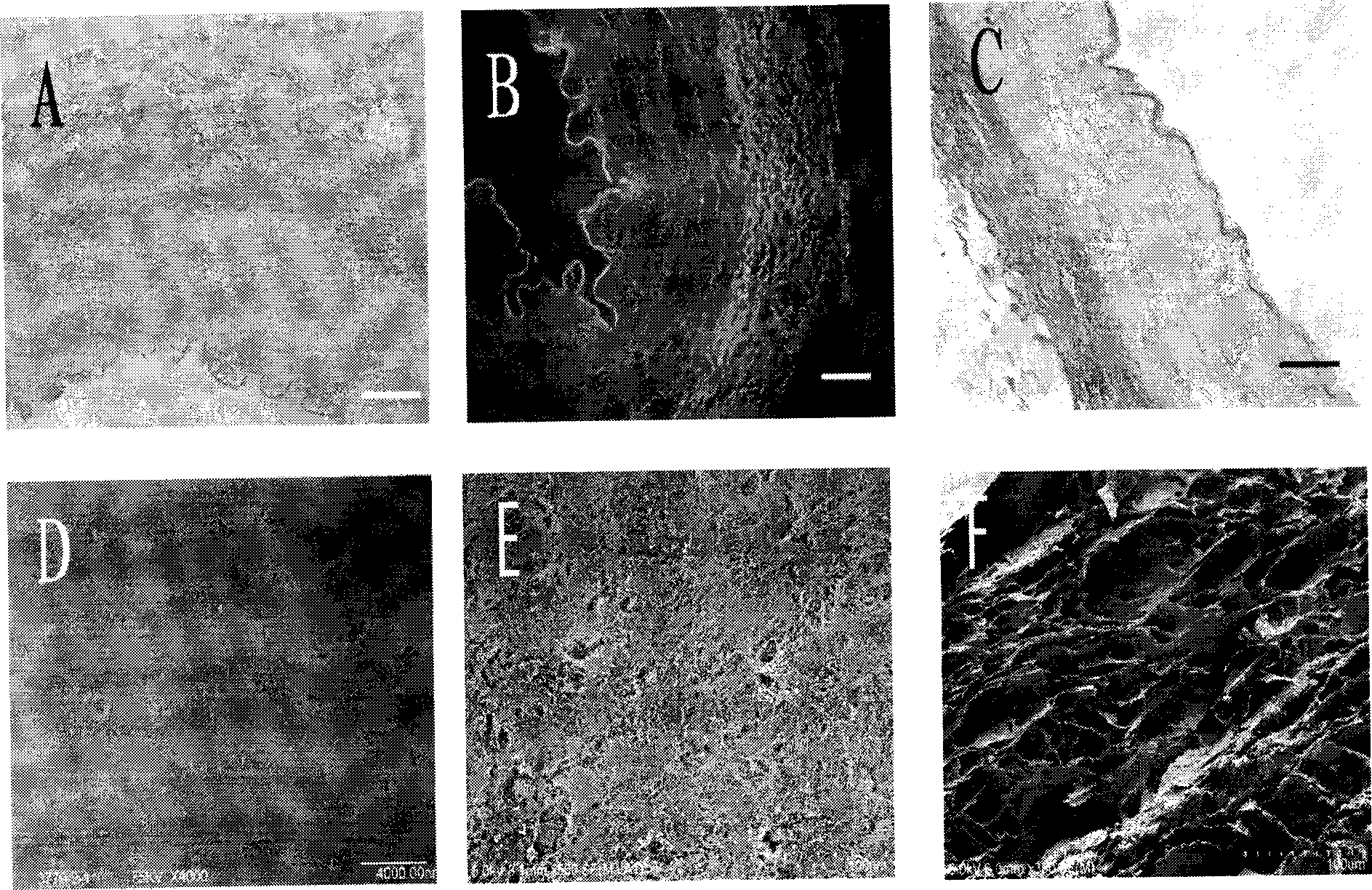
[0060] 1. Histology
[0061] Methods: Fix with 4% formaldehyde solution, observe the general structure and cell components of the acellular matrix by HE staining; observe the structural changes of collagen fibers and elastic fibers by Masson staining; observe the cell nucleus by DAPI fluorescent staining;
[0062] see results figure 1 A-1C.
[0063] 2. Electron microscope observation
[0064] Methods: The cellular components and matrix components of the acellular matrix were observed by transmission electron microscope; the changes of tissue cell structure on the surface of the tube wall were observed by scanning electron microscope.
[0065] see results figure 1 D~1F. HE( figure 1 A) and DAPI ( figure 1 B) The staining results showed that the cellular components of the porcine iliac vessels were completely removed after being sequentially treated with 1% TritonX-100, 0.1% ammonia water and 0.25% trypsin-0.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com