Human vascular endothelial cell growth inhibition factor jogged polypeptide, preparation thereof and use in targeted antineoplastic activity
A growth inhibitory factor, vascular endothelium technology, applied to human vascular endothelial cell growth inhibitory factor chimeric polypeptide and its preparation, the application field of targeted anti-tumor activity, to achieve great therapeutic value, rich application forms, tumor inhibitory activity strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Construction of chimeric protein RGD-VEGI-192A expression vector
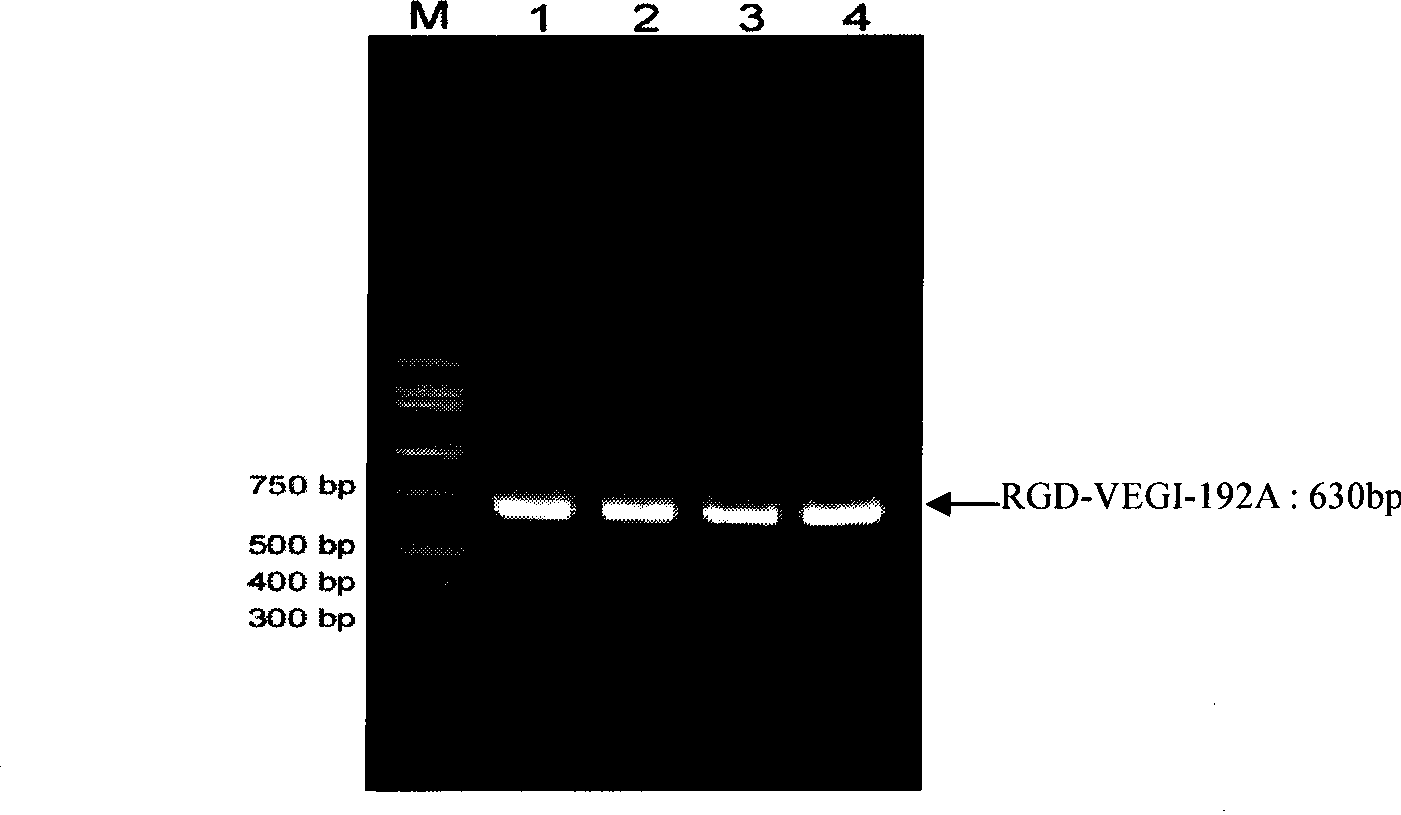
[0043] Amplified from the total RNA of human umbilical vein endothelial cells by RT-PCR technology, according to the VEGI-192A nucleotide sequence shown in SEQID NO: 1, the ACDCRGDCFCG polypeptide and the N-terminal of VEGI-192A were fused and amplified to obtain RGD-VEGI-192A target gene. The upstream primer of PCR is 5'-TTCCATATGGCTTGCGACTGCCGTGGTGACTGCTTCTGCGGTCAACTCACAAAAGGGCCGTCT-3', and the downstream primer is: 5'-CGCGGATCCCTATAGTAAGAAGGCTC CAAAGAAGGTT-3'. Both upstream and downstream primers contain Nde I restriction enzyme cutting sites at the 5' end and BamH I restriction enzyme cutting sites at the 3' end. figure 1 After PCR amplification of the RGD-VEGI-192A target gene, the PCR product is subjected to 1% agarose gel electrophoresis analysis, wherein 1, 2, 3, and 4 are PCR products, and M is a 1Kb gradient DNA molecular weight standard. figure 1 The results show that the size ...
Embodiment 2
[0045] Example 2: Auto-induced expression of RGD-VEGI-192A chimeric protein in Escherichia coli and screening of high-expression clones
[0046] 1. Automatic induction: Use the expression vector pET-30a-RGD-VEGI-192A to transform the competent cell BL21(DE3)pLysS, randomly pick 8 clone colonies into 3mL LB medium, add kanamycin to a final concentration of 50μg / mL, cultured overnight at 37°C. The bacterial cell pellet was collected by centrifugation the next day, and the recombinant expression was analyzed by SDS-PAGE.
[0047] 2.1000× trace metal solution: 0.05M FeCl 3 ;-0.12M HCl; 0.02M CaCl 2 ;0.01M MnCl 2 -4H 2 O; 0.01M ZnSO 4 -7H 2 O; 0.002M CoCl 2 -6H 2 O; 0.002M CuCl 2 -2H 2 O; 0.002M NiCl 2 -6H 2 O; 0.002M Na 2 MoO 4 -5H 2 O; 0.002M Na 2 SeO 3 -5H 2 O; 0.002MH 3 BO 3· Add double distilled water to make up to 100mL.
[0048] 3. Self-inducing expression medium: 1% tryptone (tryptone), 0.5% yeast extract (yeastextract), 25mM Na 2 HPO 4 , 25mM KH 2 ...
Embodiment 3
[0050] Example 3: Condition optimization of the automatic induction expression system
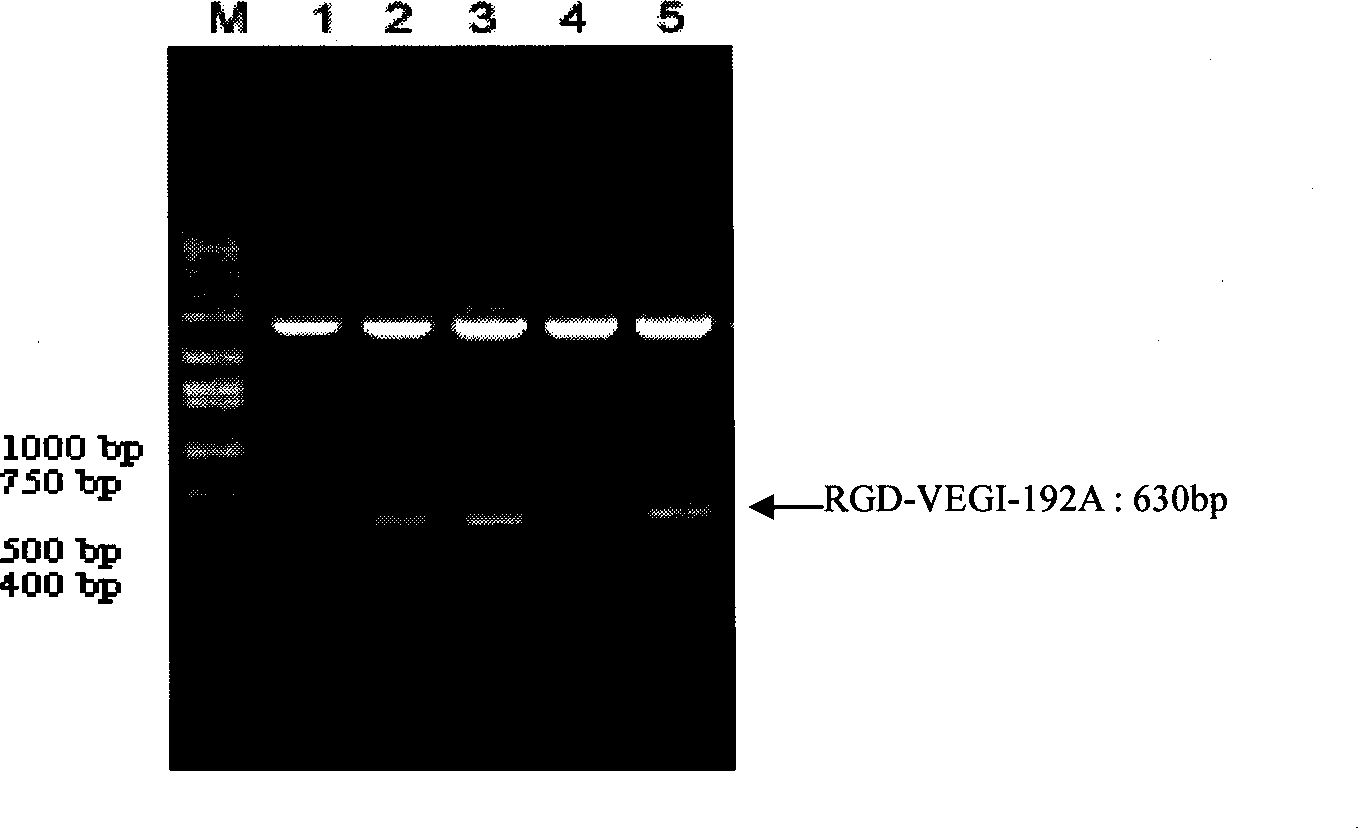
[0051] 1. Optimization of host bacteria: In order to improve the solubility of the RGD-VEGI-192A chimeric protein, we used two host bacteria, BL21(DE3)pLysS and OrigamiB(DE3), in which the OrigamiB(DE3) host bacteria had mutations in trxB and gor genes, Thereby providing balanced redox potential for the recombinant protein in the host bacteria to facilitate the correct spatial folding of the protein. According to the above image 3As a result, the yield of recombinant protein of RGD-VEGI-192A chimeric protein auto-induced in the two host bacteria is not much different, but in the host bacteria OrigamiB (DE3) auto-induced system in the background protein is slightly smaller, so we finally The host bacterium was selected as OrigamiB(DE3) #1 high-expressing clone colony, the culture time was 16 hours, the culture temperature was 25°C, and the target protein was prepared by high-density cultur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com