Use of ethyl malonyl silybin derivant in preparing antioxidant medicine
A technology of ethylmalonyl and silybin ester, which is applied in the field of medicine, can solve problems such as oxidative damage, achieve the effects of inhibiting lipid peroxidation, powerfully scavenging free radicals, and facilitating large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
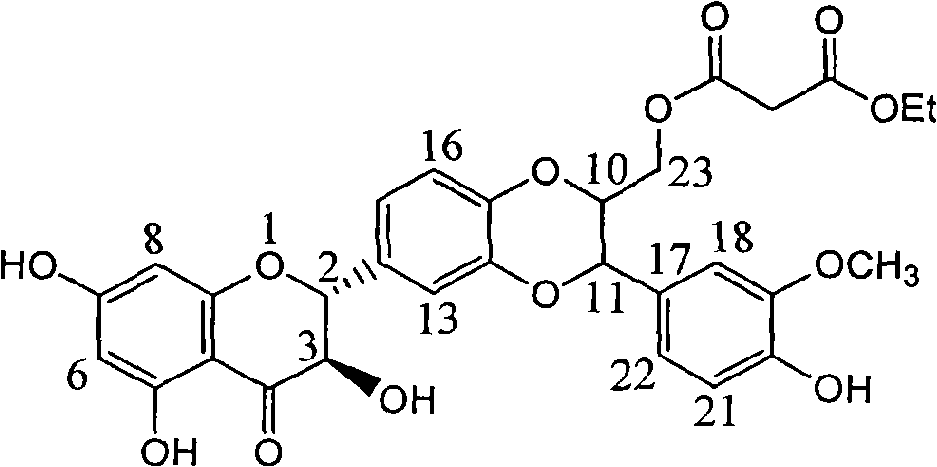
[0016] Example 1 : Compound malonic acid [3-(4-hydroxy-3-methoxyphenyl)-6-(2,3-dihydro-3,5,7-trihydroxy-4-oxo-benzopyran -2) Preparation of -2,3-dihydro-1,4-benzodioxane-2]-methyl ethyl ester
[0017]
[0018] Add 1 gram of silibinin (purchased from Liaoning Panjin Tianyuan Pharmaceutical Co., Ltd., HPLC detection purity 98%), 0.56 gram of monoethyl malonate and 1.6 gram of triphenylphosphine in the dry reaction bottle, use 20 Dissolve milliliters of anhydrous tetrahydrofuran, add 1 gram of diethyl azodicarboxylate, stir at room temperature for 10 hours after the addition is complete, distill off the solvent under reduced pressure, add 5 milliliters of chloroform, filter to remove the white solid, and the mother liquor is subjected to column chromatography to obtain shallow 0.46 g of yellow powder, yield 37%.
[0019] R f (chloroform / ethyl acetate / acetic acid=50:1:0.25):0.11; H NMR 1 HNMR (400MHz, deuterated chloroform) δ: 1.28 (triplet, J=7.2Hz, 3H, CH 3 ), 3.41 (sing...
Embodiment 2
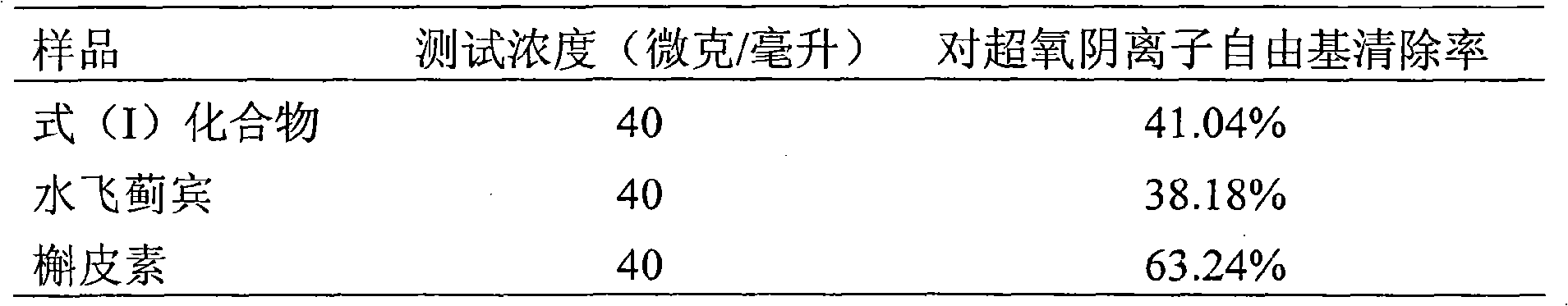
[0026] Example 2 Malonic acid [3-(4-hydroxy-3-methoxyphenyl-6-(2,3-dihydro-3,5,7-trihydroxy-4-oxo-benzopyran-2) In vitro scavenging activity of -2,3-dihydro-1,4-benzodioxane-2]-methyl ethyl ester
[0027] 2.1 Experimental materials and samples
[0028] 2.1.1 Experimental reagents:
[0029] 2.1.1.1 Phenazine methosulfate (PMS), nitrobluetetrazolium (NBT), and phenanthrozine (ferrozine) were purchased from Sigma;
[0030] 2.1.1.2 Quercetin (quercetin) was provided by the Laboratory of Traditional Chinese Medicine and Natural Medicine, School of Pharmacy, Zhejiang University (purity: 99%); silybin (Silybin) was purchased from Panjin Tianyuan Pharmaceutical Co., Ltd., Liaoning, and detected by HPLC 98% purity.
[0031] 2.1.1.3 Tris base, DMEM medium was purchased from Gibco;
[0032] 2.1.1.4 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and NADH (reduced coenzyme I) were purchased from Amresco;
[0033] 2.1.1.5 Other reagents are domestic analytical rea...
Embodiment 3
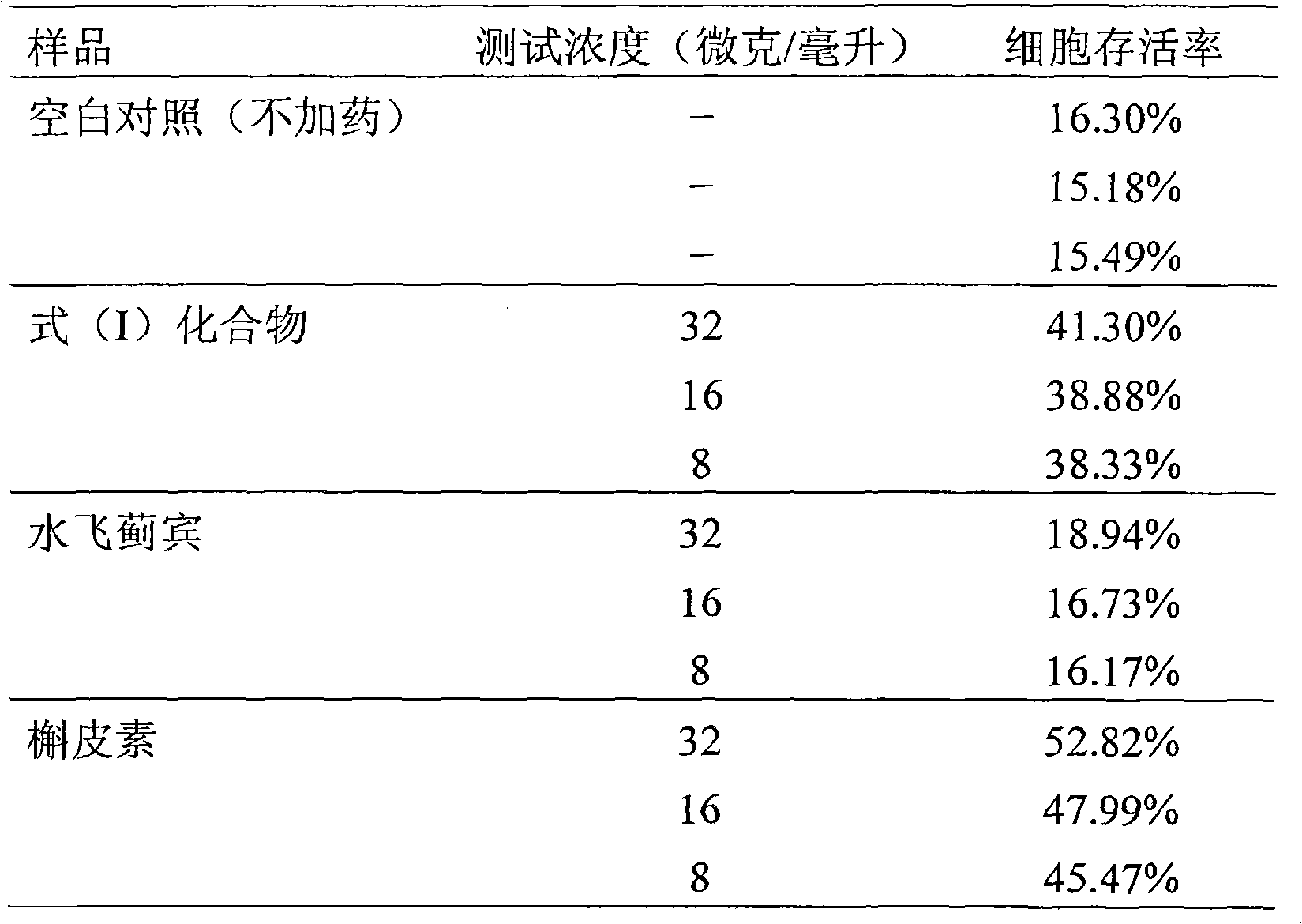
[0048] Example 3 Compound malonate [3-(4-hydroxy-3-methoxyphenyl)-6-(2,3-dihydro-3,5,7-trihydroxy-4-oxo-chromene- 2) -2,3-dihydro-1,4-benzodioxane-2]-methyl ethyl ester to hydrogen peroxide H 2 o 2 Protective activity test of induced PC12 cell injury
[0049] 3.1 Experimental materials and samples
[0050] 3.1.1 Cells: Rat adrenal pheochromoma cells (PC12) were purchased from Shanghai Institute of Cells, Chinese Academy of Sciences.
[0051] 3.1.2 Experimental reagents:
[0052] 3.1.2.1 Hydrogen peroxide (H 2 o 2 ), nitroblue tetrazolium (NBT), and phenanthrozine (ferrozine) were purchased from Sigma;
[0053] 3.1.2.2 Quercetin (quercetin) was provided by the Laboratory of Traditional Chinese Medicine and Natural Medicine, School of Pharmacy, Zhejiang University (purity: 99%); silybin (Silybin) was purchased from Liaoning Panjin Tianyuan Pharmaceutical Co., Ltd., and detected by HPLC 98% purity.
[0054] 3.1.2.3 Tris base, DMEM medium was purchased from Gibco;
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com