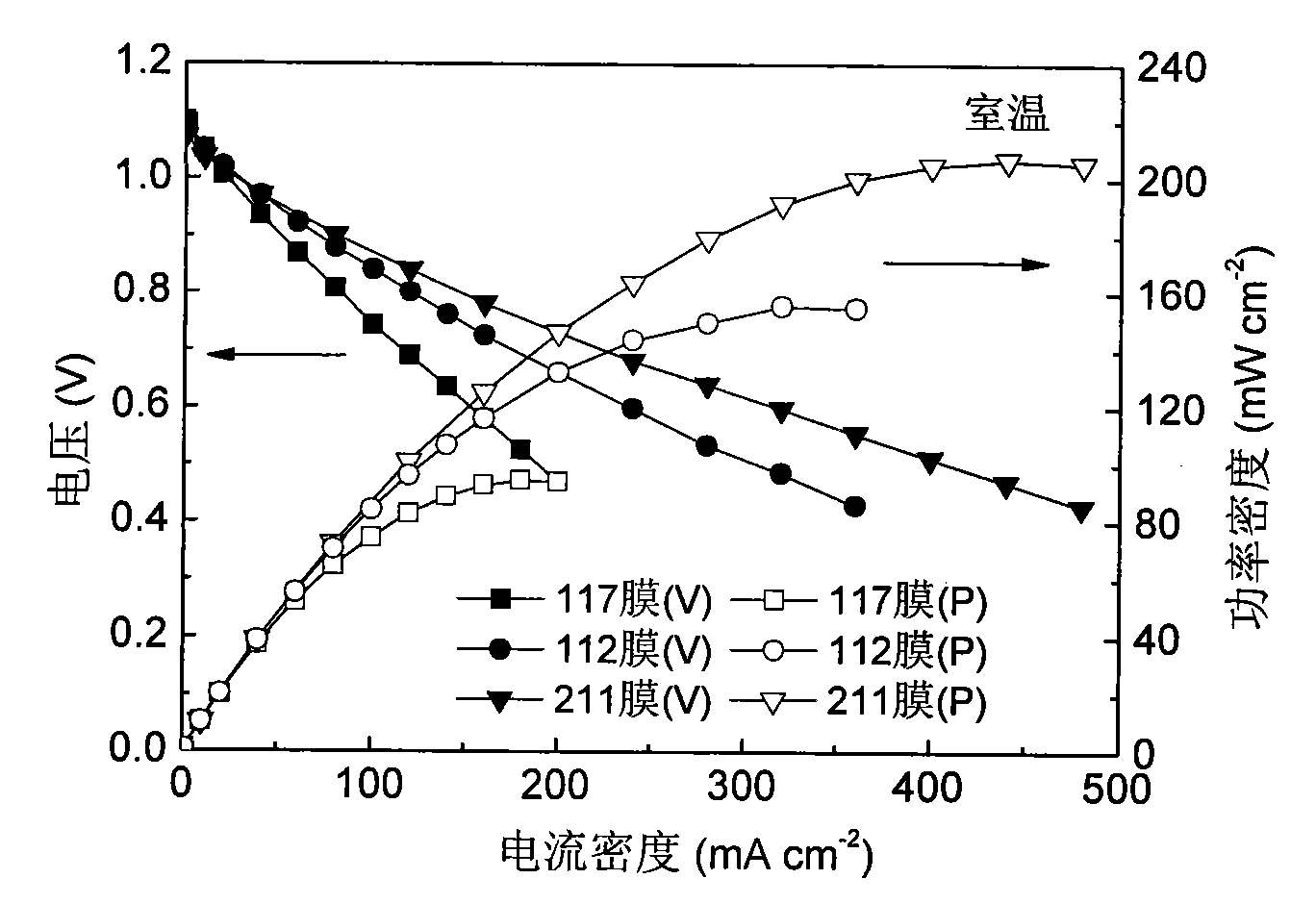
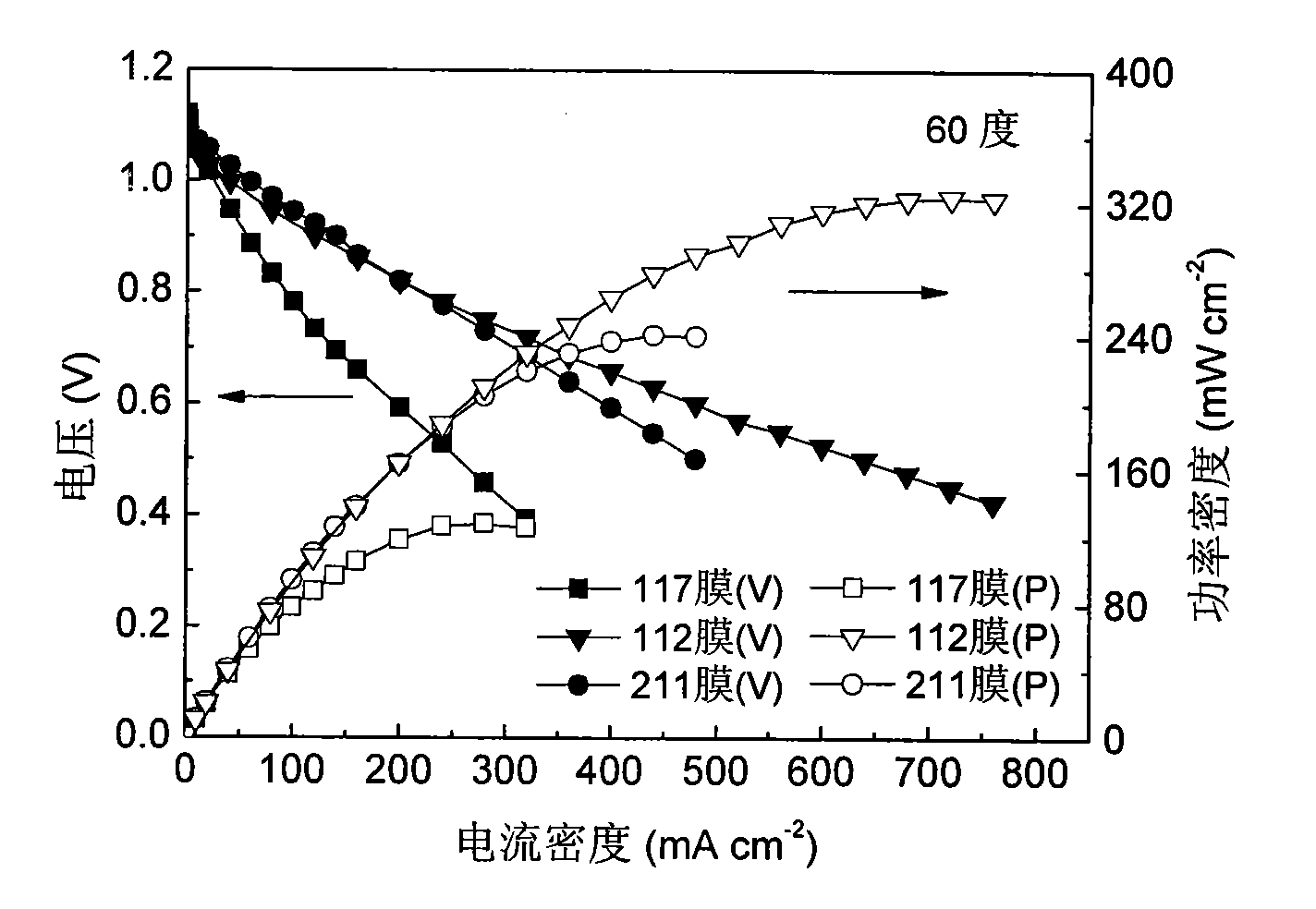
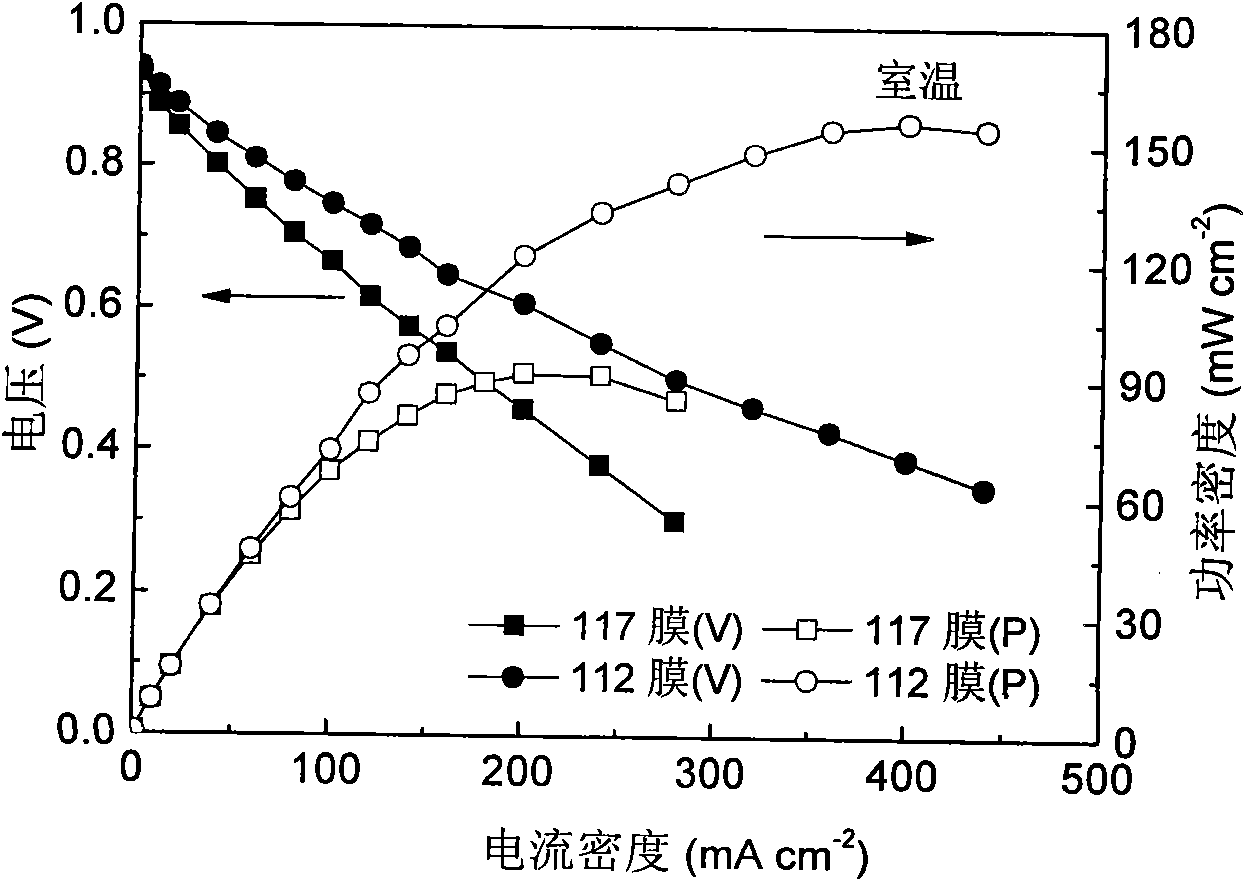
Fuel cell using conductive polymer modified carbon based cobaltous hydroxide composite catalyst
A conductive polymer, composite catalyst technology, applied in organic compound/hydride/coordination complex catalysts, solid electrolyte fuel cells, physical/chemical process catalysts, etc., can solve the problems of less research, poor catalyst performance, etc. Low cost and favorable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 10 g of carbon material to 100 ml of methanol to form a suspension, add 2 g of glacial acetic acid and stir at room temperature for 20 min, and adjust the pH to 1.0. Then add 2.5g pyrrole monomer and stir for 7min, then add 30ml 3wt.%FeCl 3 The aqueous solution was used as the initiator of the polymerization reaction, and stirred at room temperature for 3h. The product was washed with warm deionized water, and dried under vacuum at 90° C. for 12 hours to obtain a polypyrrole-modified carbon material with a polypyrrole content of 20 wt.%.
Embodiment 2
[0029] Add 10 grams of carbon material to 300 ml of water to form a suspension, add 5 g of glacial acetic acid and stir at room temperature for 20 minutes, and adjust the pH to 3.0. Then add 2g of pyrrole monomer and stir for 5min, then add 10ml of 30wt.%H 2 o 2 As a polymerization initiator, stir at room temperature for 3h. The product was washed with warm deionized water, and dried under vacuum at 90° C. for 12 hours to obtain a polypyrrole-modified carbon material with a polypyrrole content of 15 wt.%.
Embodiment 3
[0031] Add 10 grams of carbon material to 150 ml of water to form a suspension, add 2.5 g of glacial acetic acid and stir at room temperature for 20 minutes, and adjust the pH value to 3.0. Then add 2g thiophene monomer and stir for 10min, then add 10ml 10wt.% H 2 o 2 As a polymerization initiator, stir at room temperature for 3h. The product was washed with warm deionized water, and dried under vacuum at 90° C. for 12 hours to obtain a polypyrrole-modified carbon material with a polythiophene content of 15 wt.%.
[0032] Take 2.7 grams of polypyrrole-modified carbon material and pour it into a three-necked flask, add 40.5ml of water, quickly add 10 grams of 5.4wt.% cobalt chloride aqueous solution, heat to 80°C under reflux, and stir for 30 minutes. Slowly add 300ml reducing agent (basic sodium borohydride solution, containing 2.5-20wt.% NaBH4 and 5-20wt.% NaOH, the balance being water), vigorously stirred for 30 minutes, cooled naturally, filtered and washed, and dried in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com