Anti-I type Shiga toxin IgY antibody as well as preparation method and use thereof
A technology of Shiga toxin, type I, applied in the preparation of the antibody, the field of IgY antibody specific for type I Shiga toxin, can solve problems such as no reports, and achieve the effect of inhibiting the toxicity of Shiga toxin and having a good application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
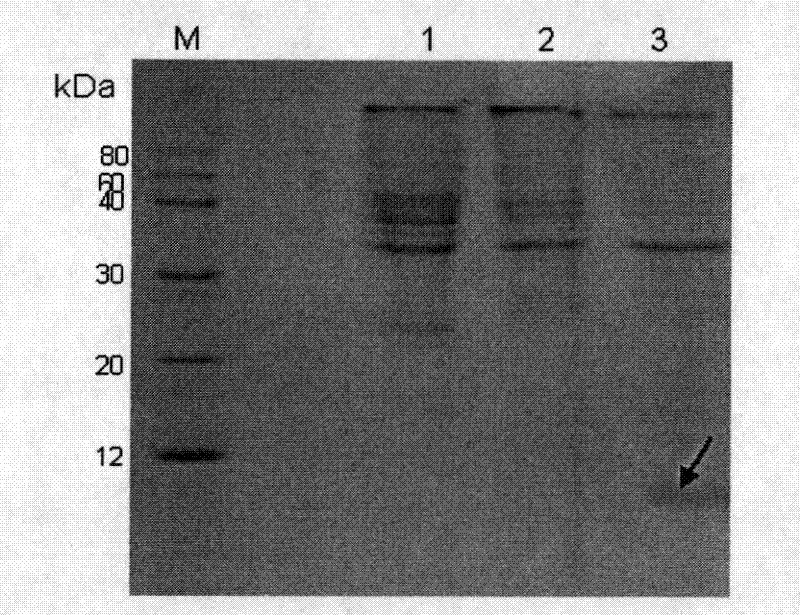
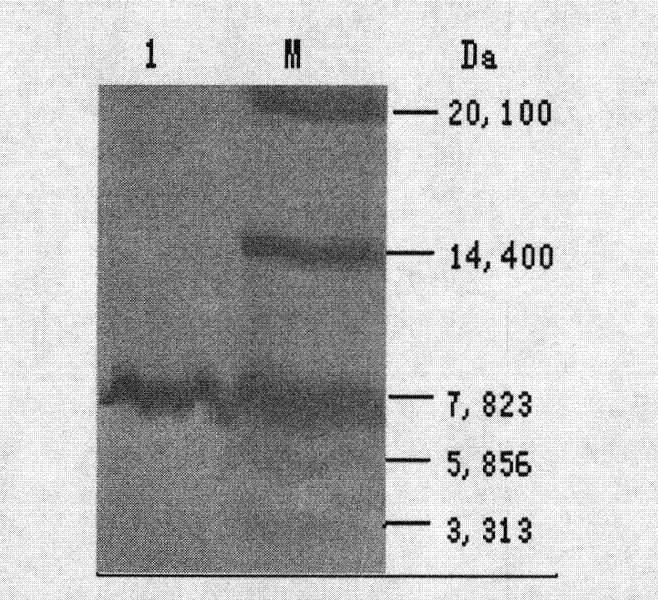
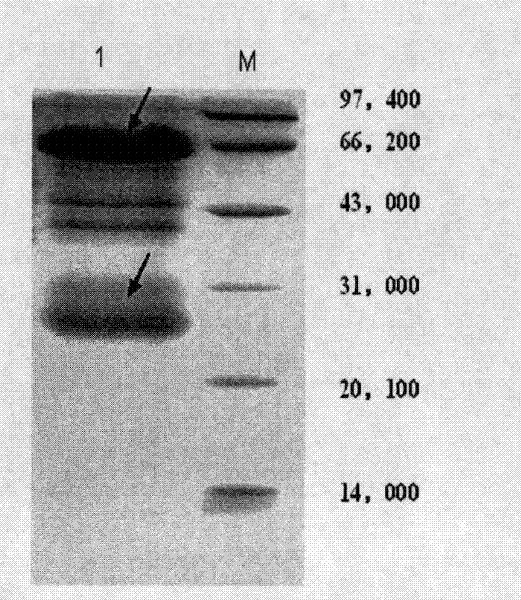
[0018] Example 1 Expression and purification of recombinant Stx1B
[0019] 1. Material
[0020] 1.1 strain
[0021] The engineered strain pBV220-stx1b / DH5α was constructed by our laboratory for the expression of foreign protein Stx1B.
[0022] 1.2 Reagents
[0023] Ion exchange chromatography column Sepharose TM HP HTrap TM And Sephacry S-100 gel column (26 / 100) were purchased from GE Company.
[0024] 2. Methods and results
[0025] 2.1 Induced expression of recombinant StxB in E. coli
[0026] Inoculate the engineered strain pBV220-stx1b / DH5α into LB liquid medium containing 50μg / mL ampicillin, shake overnight at 37°C, inoculate the LB liquid medium at 1:100 the next day, and continue shaking culture at 37°C until the bacterial solution Concentration reaches OD 600 For 0.8~1.0, quickly increase the temperature to 41℃ and continue to incubate for 5 hours. Centrifuge at 5000g for 10 min at 4°C, wash the bacteria once with pre-cooled PBS, collect the bacteria by centrifugation, and w...
Embodiment 2
[0032] Example 2 Preparation of anti-type I Shiga toxin IgY antibody
[0033] 1. Material
[0034] Laying chickens were provided by Beijing Experimental Animal Center, and both complete and incomplete adjuvants were purchased from Sigma.
[0035] 2. Methods and results
[0036] 2.1 Animal immunity (Table 1)
[0037] Mix the purified recombinant Stx1B with Freund’s adjuvant (lanolin: liquid paraffin = 1:3, autoclaved for later use) in equal volume, stir well and immunize SPF-2 layer hens, add 1mg / ml BCG vaccine for the first immunization (China Institute for the Control of Pharmaceutical and Biological Products).
[0038] Table 1 Animal Immunization Implementation
[0039] Immunization time
Immunization dose
Immune pathway
0d
1mg / piece
Freund's complete adjuvant
Subcutaneous and intramuscular injections
14d
1mg / piece
Freund's incomplete adjuvant
Subcutaneous and intramuscular injections
28d
0.5mg / piece
Freund's incomplete adjuvant
Subcutaneous and ...
Embodiment 3
[0046] Example 3 Application of IgY antibody in Shiga toxin detection
[0047] 1. Material
[0048] BIAcore 3000 biosensor system, CM5 chip, coupling reagents, working fluid, etc. are all products of GE, and anti-IgY-HRP was purchased from SIGMA. Enzyme-linked plates and color reagents were purchased from Bioconn.
[0049] 2. Methods and results
[0050] 2.1 IgY antibody and Stx1B binding specificity (ELISA)
[0051] Enzyme-linked plates are coated with antigens such as type I Shiga toxin (Stx1), type II Shiga toxin (Stx2), tetanus toxin (Tet), Staphylococcus aureus enterotoxin B (SEB), 10μg per well, ELISA method To detect the binding activity of IgY, the secondary antibody is HRP-labeled rabbit anti-chicken antibody (SIGMA). The results showed that IgY antibody can specifically recognize type I Shiga toxin and have obvious binding effect, but does not bind to other toxin antigens (tetanus toxin-Tet, Staphylococcus aureus enterotoxin B-SEB), especially type II Shiga The toxin (Stx2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com