Dehydrogenated silibinin substituted by meta-chlorobenzene formoxyl, preparation method and pharmaceutical applications thereof
A technology of dehydrosilibinin and chlorobenzoyl, which is applied in the field of medicine, can solve the problems of water solubility and bioavailability limiting the drug market, etc., and achieve the effect of short steps, simple method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
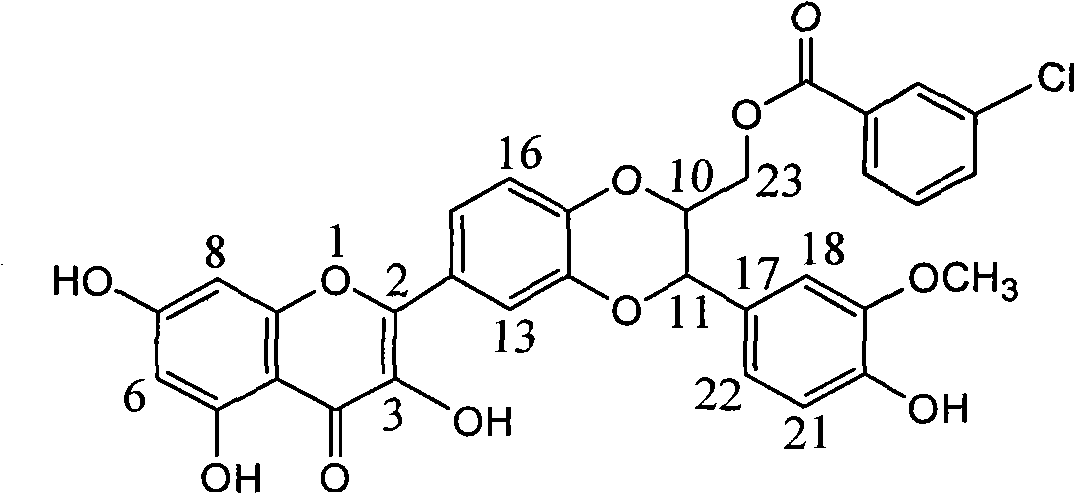
[0015] Example 1 : Formula (I) compound m-chlorobenzoic acid [3-(4-hydroxy-3-methoxyphenyl)-6-(3,5,7-trihydroxy-4-oxo-benzopyran-2 )-2,3-dihydro-1,4-benzodioxane-2]-methyl ester
[0016]
[0017] 1.12, Preparation of 3-dehydrosilibinin:
[0018] Add 1.02 grams of silybin, 10 grams of potassium acetate, and 50 milliliters of glacial acetic acid into the dry reaction bottle, and then add 50 milliliters of glacial acetic acid dissolved in 1 gram of iodine under ice cooling. Heat to reflux for 5 hours. After cooling down, 50 ml of ice water was added, extracted with ethyl acetate (20 ml x 4 times), the organic layers of the extracts were combined, washed with water, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to obtain a reddish-brown solid, which was added to 50 ml of ethanol solution containing 6 ml of hydrochloric acid. Heat to reflux for 3 hours. After lowering the temperature, a yellow solid was precipitated, and recrystall...
Embodiment 2
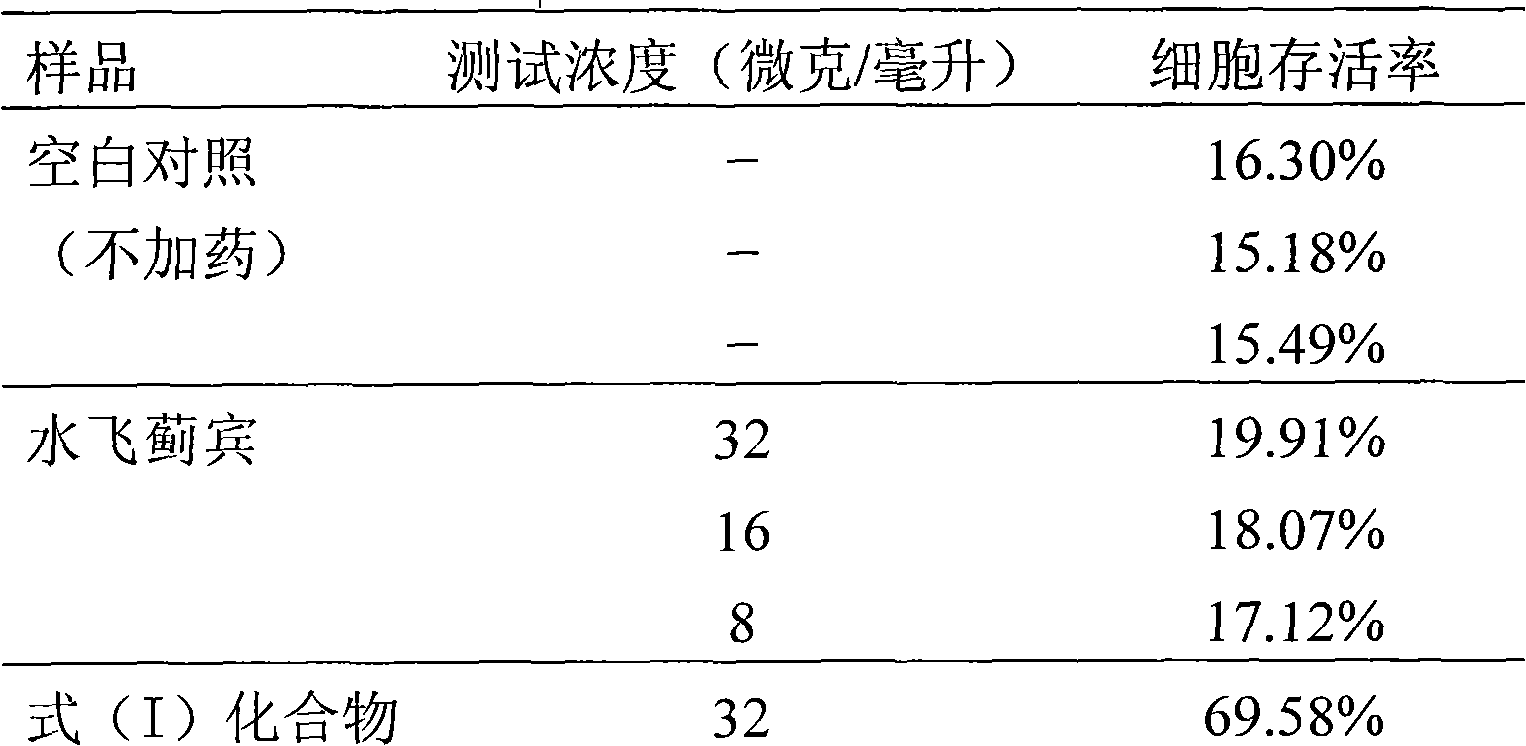
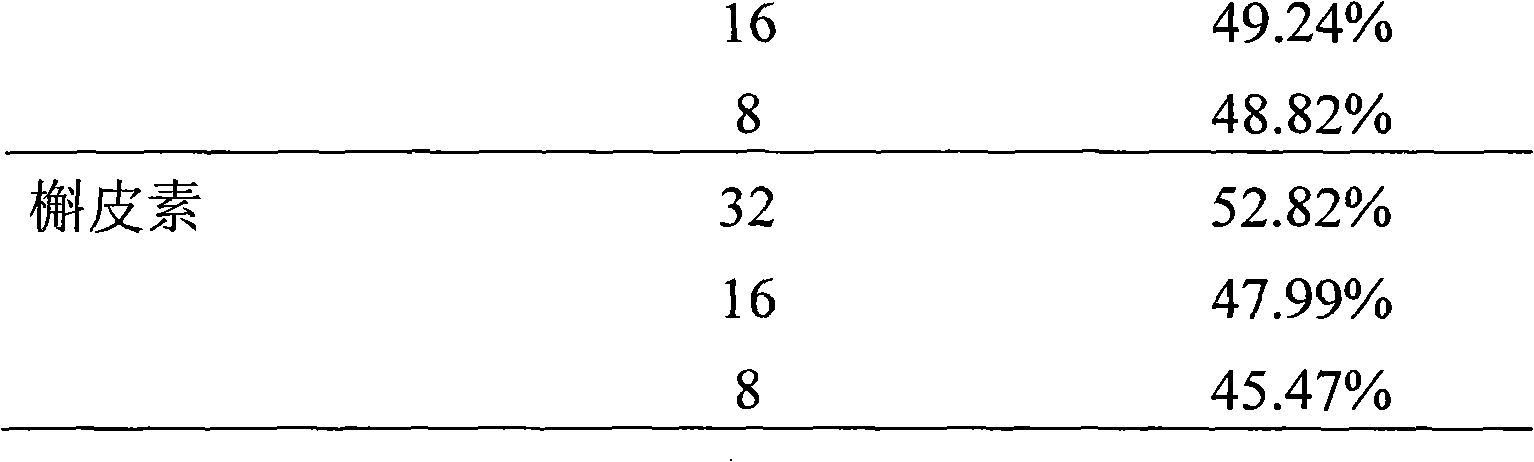
[0028] Example 2 : Formula (I) compound m-chlorobenzoic acid [3-(4-hydroxy-3-methoxyphenyl)-6-(3,5,7-trihydroxy-4-oxo-benzopyran-2 )-2,3-dihydro-1,4-benzodioxane-2]-methyl ester to hydrogen peroxide H 2 o 2 Protective activity test of induced PC12 cell injury
[0029] 2.1 Experimental materials and samples
[0030] 2.1.1 Cells: rat adrenal pheochromocytoma (PC12) cells were purchased from Shanghai Institute of Cells, Chinese Academy of Sciences.
[0031] 2.1.2 Experimental reagents:
[0032] 2.1.2.1 Hydrogen peroxide (H 2 o 2 ), nitroblue tetrazolium (NBT), and phenanthrozine (ferrozine) were purchased from Sigma;
[0033] 2.1.2.2 Quercetin (quercetin) was provided by the Laboratory of Traditional Chinese Medicine and Natural Medicine, School of Pharmacy, Zhejiang University (purity: 99%); Silybin (Silybin) was purchased from Panjin Tianyuan Pharmaceutical Co., Ltd., Liaoning, and detected by HPLC 98% purity.
[0034] 2.1.2.3 Tris base, DMEM medium was purchased from...
Embodiment 3
[0061] Example 3 : Formula (I) compound m-chlorobenzoic acid [3-(4-hydroxy-3-methoxyphenyl)-6-(3,5,7-trihydroxy-4-oxo-benzopyran-2 )-2,3-dihydro-1,4-benzodioxane-2]-methyl ester inhibits the activity of free radical-induced lipid peroxide generation
[0062] 3.1 Experimental principle: Lipid peroxidation is the product of free radicals acting on polyunsaturated fatty acids, and the content is positively correlated with the generation of free radicals. Because the body has anti-oxidant enzymes and non-enzyme protection, free radicals are continuously generated was continuously removed. With the growth of age, the antioxidants in the body continue to decline, the ability to scavenge free radicals gradually weakens, the lipid peroxidation increases, and the lipid peroxidation products increase. Therefore, the determination of the anti-lipid peroxidation effect of samples is one of the important indicators for screening anti-oxidation and anti-aging drugs. Vitamin C-Fe was use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com