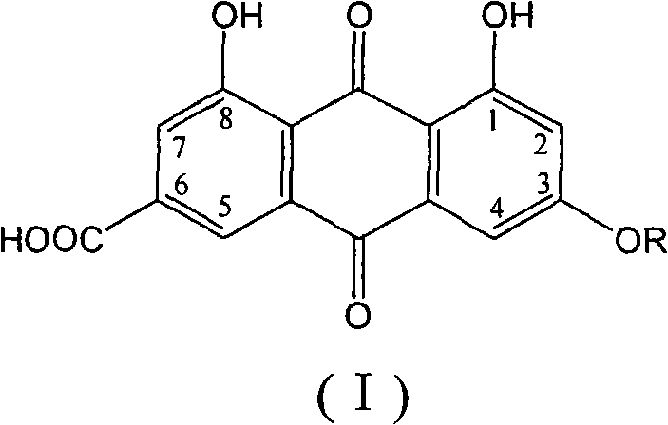
3-alkoxyl-6-carboxyl rhein or officinal salt thereof and preparation method and application thereof
A technology of alkoxyemodin ether and alkoxyl, which is applied in the field of emodin derivatives, can solve the problems that have not yet been seen in the research reports on the preparation method and application of 3-alkoxyl-6-carboxyrhein, and achieve Good development and application prospects, high product yield, strong antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1,3-alkoxy-6-carboxyrhein
[0030] Include the following steps:
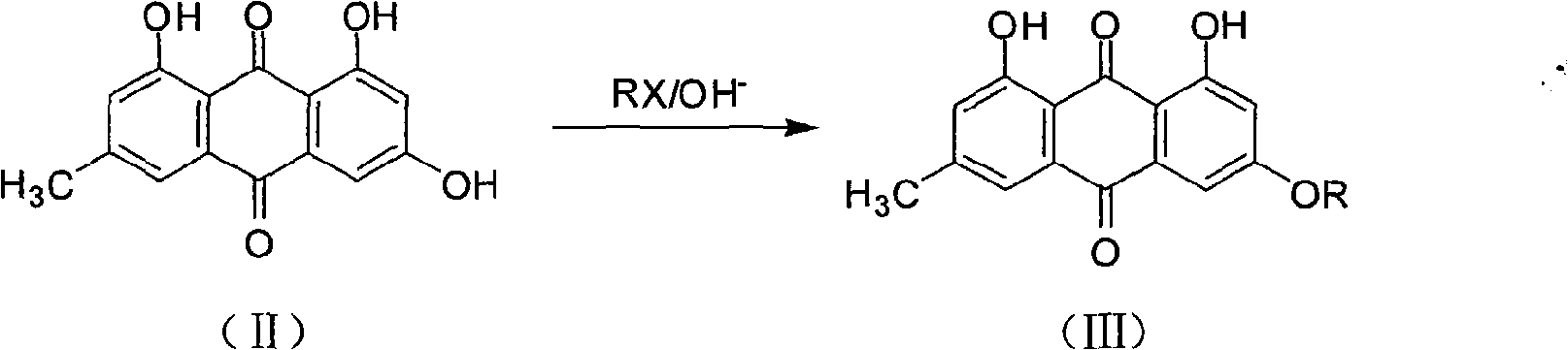
[0031] The preparation of a, 3-alkoxyemodin ether
[0032] Weigh 5mmol of emodin and 6mmol of sodium carbonate, add 50ml of DMF, stir to dissolve, add dropwise a mixture of 5-15mmol of haloalkane and 50ml of DMF. ) method to monitor the reaction process, after the reaction, adjust the pH to 8~9 with the sodium carbonate solution of 2.5% with the mass percentage concentration, suction filtration, the filter cake is washed with the sodium carbonate solution of 2.5% with the mass percentage concentration, ethyl acetate weighs Crystallize and dry to obtain 3-alkoxyemodin ether. See Table 1 for the preparation of some 3-alkoxyemodin ethers.
[0033] Table 1, the preparation situation of part 3-alkoxyemodin ether
[0034]
[0035] Continued Table 1
[0036]
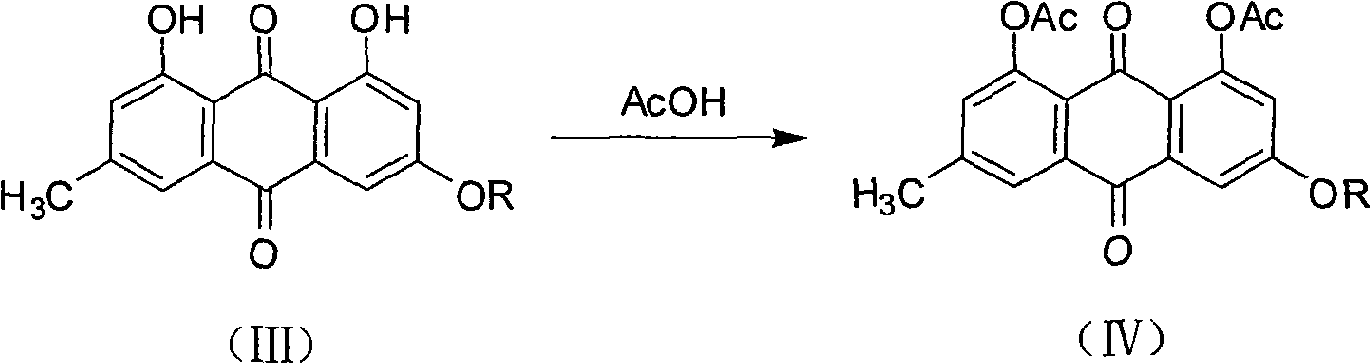
[0037] b. Preparation of 3-alkoxy-1,8-diacetylemodin ether
[0038] Weigh 0.5mmol of the 3-alkoxyemodin ether ob...
Embodiment 2
[0055] The preparation of embodiment 2,3-alkoxy-6-carboxyrheinate
[0056] Weigh 0.005 mol of 3-alkoxy-6-carboxyrhein obtained in Example 1, add equimolar alkali solution, and stir for reaction to obtain 3-alkoxyl-6-carboxyrheinate. See Table 5 for the preparation of some 3-alkoxyl-6-carboxyrheinates.
[0057] Table 5, the preparation situation of part 3-alkoxy-6-carboxyrheinate
[0058]
[0059]According to the method described in Example 1 or 2, the 3-alkoxy-6-carboxyrhein of the present invention or its pharmaceutically acceptable salt can be obtained by using a haloalkane with a carbon number of 1 to 24, and the structure of the product is determined by ultraviolet spectroscopy. (UV), infrared spectroscopy (IR) and nuclear magnetic resonance spectroscopy (NMR) for confirmation. The following are some structural characterization results of 3-alkoxy-6-carboxyrhein:
[0060] 3-butoxy-6-carboxyrhein: orange crystals, m.p.260~261.5℃; IR(KBr)υ: 3467, 3100, 2961, 1702, 1626...
experiment example 1
[0065] Antibacterial activity detection of experimental example 1, 3-alkoxy-6-carboxyrhein or its pharmaceutically acceptable salt
[0066] Emodin or 3-alkoxyl-6-carboxyrhein or 3-alkoxyl-6-carboxyrheinate were dissolved in water respectively to make a solution with a concentration of 1000ppm / ml as the test solution; 0.5ml of product solution, half-diluted with MH broth, the dilutions were 1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128, 1:256, 1:512; at the same time, a negative control without adding the test solution was set; each group was added with a titer of 10 8 cfu / ml Staphylococcus aureus or Escherichia coli bacterial solution 0.05ml, mixed evenly, cultured at 37°C for 24 hours, and tested the minimum inhibitory concentration (MIC).
[0067] The results are shown in Table 6. The minimum inhibitory concentrations of 3-alkoxyl-6-carboxyrhein are lower than emodin, especially 3-octyloxyl-6-carboxyrhein or its pharmaceutically acceptable salts. The bacterial concentration is 1 / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com