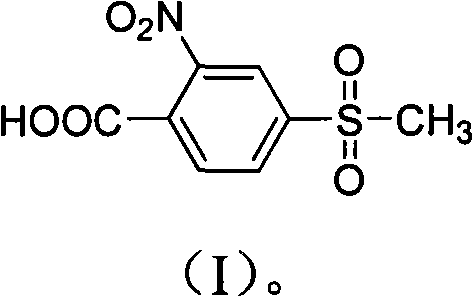
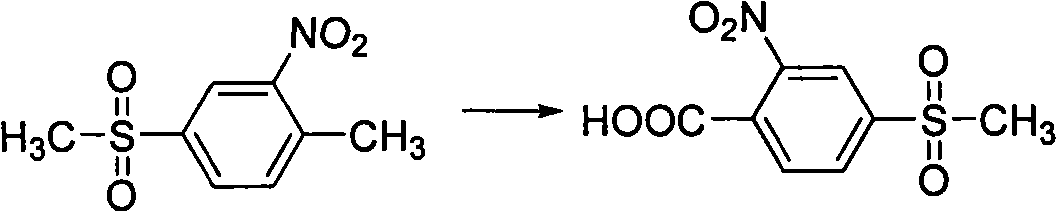Preparation method of 2-nitryl-4-thiamphenicol benzoic acid
A technology of methylsulfonyl benzoic acid and methylsulfonyl benzonitrile, which is applied in the field of preparation of 2-nitro-4-methylsulfonyl benzoic acid, can solve problems such as complicated reaction steps, and achieves high product purity, simple process, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] According to the present invention, the preparation method of described 2-nitro-4-thiamphenicol benzoic acid comprises the following steps:
[0020] (1) Under cyanidation reaction conditions and in the presence of a catalyst, 2-nitro-4-thiamphenicol chlorobenzene is reacted with cyanide to obtain 2-nitro-4-thiamphenicol benzonitrile;
[0021] (2) The 2-nitro-4-thiamphenicol benzonitrile obtained in the step (1) is subjected to a hydrolysis reaction.
[0022] The preparation route of 2-nitro-4-thiamphenicol benzoic acid provided by the invention can be represented by following reaction formula:
[0023]
[0024] According to the present invention, in the step (1), 2-nitro-4-thiamphenicol chlorobenzene and cyanide can react according to the stoichiometric ratio, in order to make the raw material 2-nitro-4-thiamphenicol chlorobenzene For a more complete reaction, the molar ratio of 2-nitro-4-thiamphenicol chlorobenzene to cyanide can be 1:1-2; preferably 1:1.1-1.5.
...
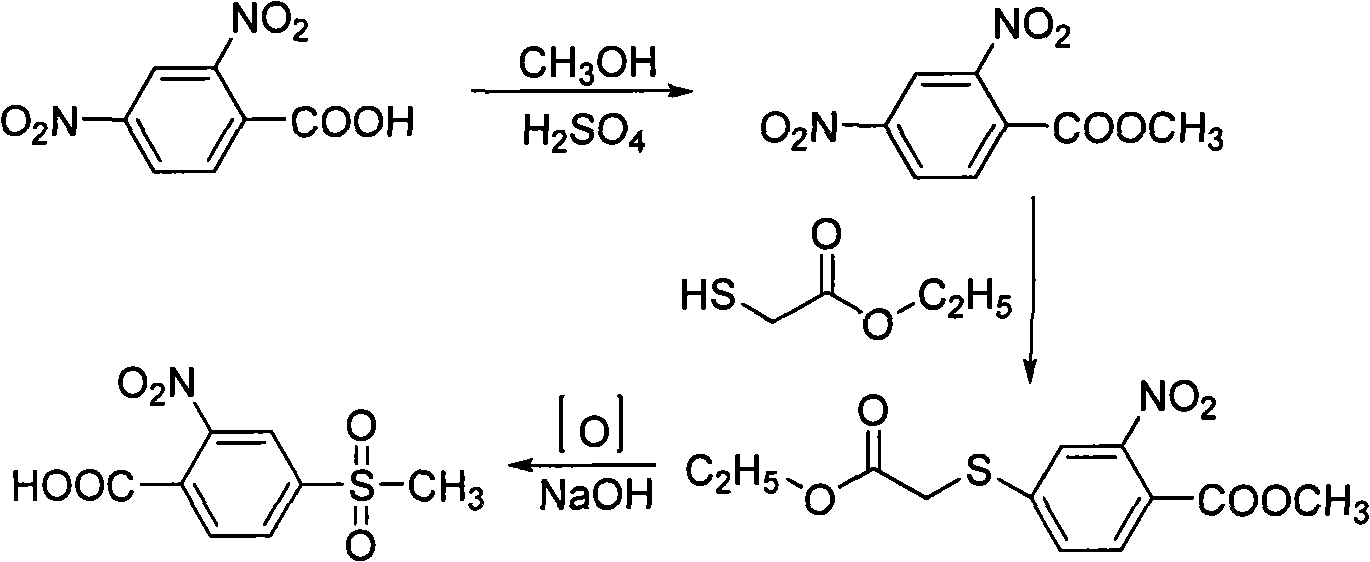
preparation Embodiment 1
[0052] This example is used to illustrate the preparation of 2-nitro-4-thiamphenicol chlorobenzene
[0053] (1) Add 174.8 grams (1.5 moles) of chlorosulfonic acid, 8.8 grams (0.15 moles) of sodium chloride and 150 milliliters of 1,2-dichloroethane in turn in the reaction flask; start stirring, heat and heat up to 55-60 ℃; then 1,2-dichloroethane solution (250 ml of 1,2-dichloroethane) containing 79.8 grams of o-chloronitrobenzene (0.5 mol, 99%) was added dropwise to the above-mentioned In the mixed solution; after the dropwise addition, keep warm for 5 hours, pour into 500 ml of ice-water mixture, stir, let stand, and separate the organic layer; dry the organic layer, and precipitate to obtain a light yellow solid 3-nitro- 4-Chlorobenzenesulfonyl chloride.
[0054] (2) Add 75.6 grams (0.6 moles) of anhydrous sodium sulfite, 400 milliliters of water and 84 grams (1.0 moles) of anhydrous sodium bicarbonate in the reaction flask in sequence; start stirring, heat to 70 ° C, and a...
Embodiment 1
[0056] This embodiment is used to illustrate the preparation of 2-nitro-4-thiamphenicol benzoic acid
[0057] (1) Under the protection of nitrogen gas, 130 grams of N-methylpyrrolidone and 22.8 grams (0.1 moles, 98.7%) of the 2-nitro-4-thiamphenicol prepared in Preparation Example 1 were successively added to the reaction flask Chlorobenzene, 11.1 grams (50 mmoles) of nickel bromide and 9.0 grams (0.1 moles) of cuprous cyanide, started stirring, heated to 160 ° C, stirred for 4 hours; then added 500 milliliters of ether and 150 milliliters of water, stirred, Stand to separate layers, dry the organic phase with anhydrous sodium sulfate, and remove the solvent to obtain 20.4 grams of solid;
[0058] (2) Add 20.4 grams of the solid obtained in step (1) and 50 milliliters of ethylene glycol successively to the reaction flask, start stirring, add 50 milliliters of water, 14.4 grams (0.36 moles) of sodium hydroxide, heat to 110° C., and keep warm 3 hours, cooled to room temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com