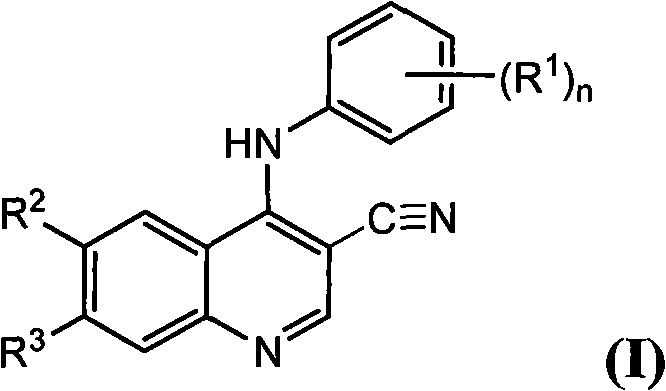
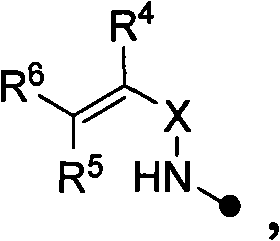
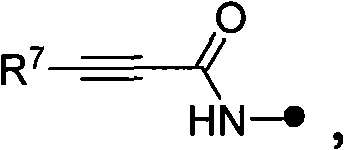
Quinoline as well as pharmaceutical composition and use thereof
A derivative, quinoline technology, applied in the field of quinoline derivatives, can solve problems such as poor prognosis and achieve excellent therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0192]
[0193] Make 1,1-dimethyl-2-propyl acetate (2-methyl-3-butyn-2-yl acetate) (51.5g, 408.2mmol), copper (I) chloride (2.02g , 20.4mmol), triethylamine (56.6mL, 408.2mmol) and 1-methylpiperazine (54.3mL, 489.9mmol) in THF (480mL) were reacted under reflux for 2 hours. The reaction solution was concentrated, tert-butyl methyl ether (200 mL) was added to the residue, and the product was extracted with dilute hydrochloric acid. While stirring the extract under ice cooling, 6N aqueous sodium hydroxide solution was added until the aqueous layer became basic, and extracted with dichloromethane (500 mL×1, 150 mL×3). The extract was washed with 14% ammonia water and then with saturated brine, then dried and concentrated. The resulting dark brown solid was purified by sublimation (60°C / 5-6 Torr) to obtain the title compound as colorless crystals (49.07 g, 72%).
[0194]
[0195] 4a: 1 H NMR (CDCl 3 ) δppm: 1.40(s, 6H), 2.28(s, 1H), 2.28(s, 3H), 2.49(t, 4H), 2.69(t, 4H). ...
Synthetic example 2
[0196]
[0197] 1) Dissolve 96.5mmol of 4,7-dichloro-6-nitro-3-cyanoquinoline and 14.05g (96.5mmol) of 3-chloro-4-fluoroaniline in 900ml of 2-propanol, nitrogen Reflux for 3.5h under protection. TLC (ethyl acetate:n-hexane=1:1) showed no starting point. Stir at room temperature overnight, filter and wash with 2-propanol to obtain 36.5 g of 4-(3-chloro-4-fluoroanilino)-7-chloro-6-nitro-3-cyanoquinoline.
[0198] 2) In 4-(3-chloro-4-fluoroanilino)-7-chloro-6-nitro-3-cyanoquinoline (40.1mmol), 1-(1,1-dimethyl-2- Propyl)-4-methylpiperazine (4a) (10.0g, 60.1mmol), copper (I) iodide (380mg) and tetrakis (triphenylphosphine) palladium (1.39g) in DMF solution (70mL) After blowing nitrogen gas at 50°C for 15 minutes, triethylamine (13.9 mL, 100.0 mmol) was added, and stirred at an oil bath temperature of 140°C for 50 minutes. After standing to cool, the reaction solution was concentrated, and aqueous sodium bicarbonate solution (300 mL) was added. The resultant was extracted with...
Synthetic example 3
[0204]
[0205] 1) 2-cyano-3-ethoxy ethyl acrylate In a 2000ml three-necked reaction flask equipped with a fractionating column, add 280ml (2.5mol) of ethyl cyanoacetate and 833ml (5.0mol) of triethyl orthoformate , 27.8ml of acetic anhydride was reacted at 120°C for 3h, the distillate was recovered by distillation, 187ml of absolute ethanol was added after cooling, crystallized by cooling, the crude product was filtered, recrystallized with absolute ethanol after drying, and 312.2g of light yellow crystals were obtained. was 88.0%.
[0206] 2) 4-Hydroxy-6-nitro-7-bromo-3-cyanoquinoline
[0207] 0.324 mol of 3-bromo-4-nitroaniline (Mieczyslaw Makosza and Maciej Bialecki, JOrg Chem, 1998, 63, 4878-4888) and 77.2 g (0.456 mol) of 2-cyano-3-ethoxyacrylic acid ethyl The ester was dissolved in 210ml of toluene and refluxed for 16h. Cool in an ice bath, filter, and wash with ether to obtain 94.2 g of solid. The obtained solid (37.5 g, 0.123 mol) was added to a 5 L three-necked ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com