Metal carrier load gold catalyst and application thereof in preparing aldehyde or ketone by selectively oxidizing catalytic alcohol
A gold catalyst and metal carrier technology, applied in the field of catalysis, can solve the problems of high cost, many by-products, harsh reaction temperature, etc., and achieve the effects of low production cost, high activity at low temperature and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] According to the literature (Applied Catalysis A 2007, 328: 77-82), a papermaking / post-sintering technique was adopted to prepare a sintered metal fiber carrier.
[0021] The metal fibers are: Ni fiber with a diameter of 4 microns, Ni fiber with a diameter of 8 microns, copper fiber with a diameter of 8 microns, copper fiber with a diameter of 80 microns, copper fiber with a diameter of 120 microns, brass fiber with a diameter of 120 microns, and a diameter of 8 microns Stainless steel (316L) fibers, titanium fibers with a diameter of 16 microns; the length of the fibers is 2 to 5 mm.
[0022] The prepared sintered metal fiber supports are represented as S-X-Ni-4, S-X-Ni-8, S-X-Cu-Z-8, S-X-Cu-Z-80, S-X-Cu-Z-120, S-X-Cu- H-120, S-X-SS and S-X-Ti.
Embodiment 2
[0024] This example provides a preparation of a gold catalyst supported by a sintered metal fiber carrier.
[0025] Using S-X-Ni-8 as the carrier, impregnate S-X-Ni-8 with equal volumes of chloroauric acid aqueous solution containing 2 g / L, 4 g / L, 6 g / L, 8 g / L and 10 g / L of gold respectively Carrier, 400 ℃ of calcining in air after drying 2 hours, makes the gold catalyst of sintering Ni fiber support, and Au content is respectively 1% (weight), 2% (weight), 3% (weight), 4% (weight ) and 5% (weight), the catalyst product is recorded as Au-1 / S-X-Ni-8, Au-2 / S-X-Ni-8, Au-3 / S-X-Ni-8, Au-4 / S-X-Ni- 8, Au-5 / S-X-Ni-8.
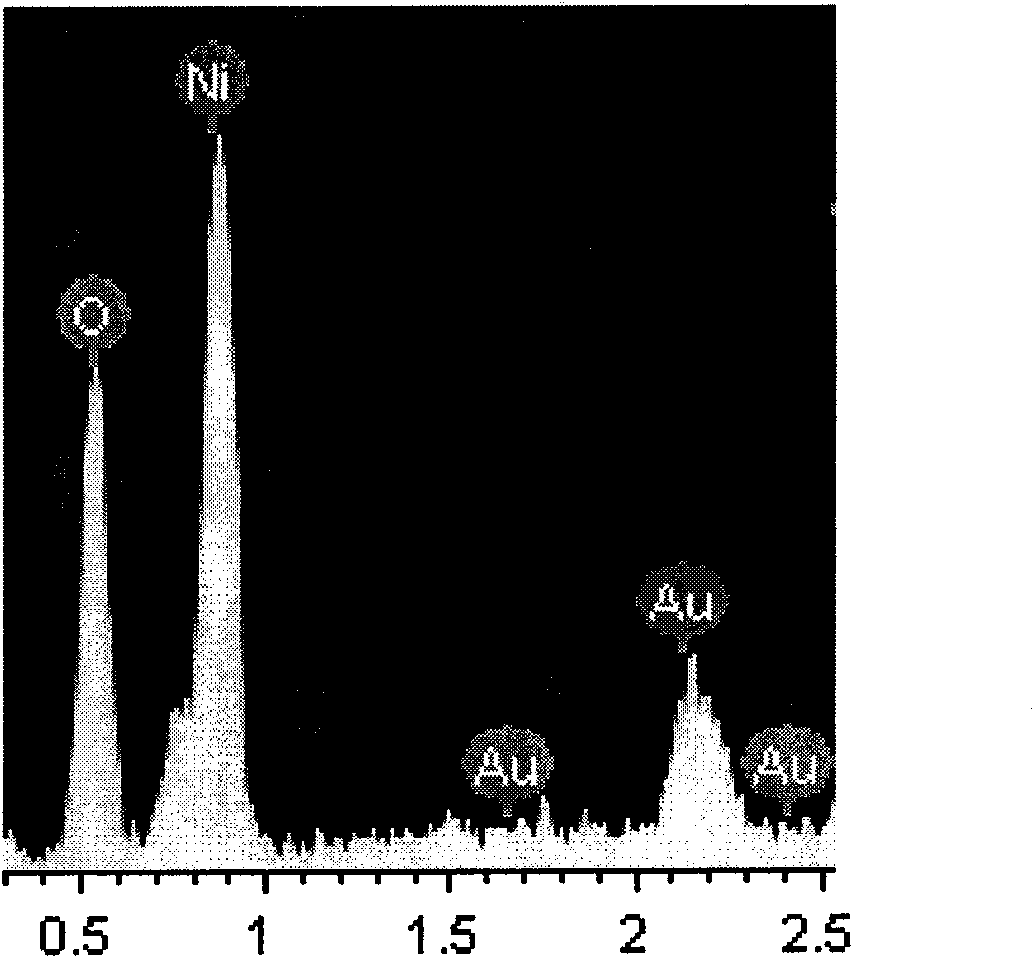
[0026] Optical photo of Au-3 / S-X-Ni-8 for reference figure 1 , scanning electron microscope (SEM) photos and energy spectrum (EDX) photos refer to figure 2 with image 3 .
Embodiment 3
[0028] This example provides the preparation of a gold catalyst supported on a sintered metal fiber carrier.
[0029] Using X-Ni-8 as the carrier, the X-Ni-8 carrier is impregnated with an equal volume of chloroauric acid aqueous solution containing 6 g / L of gold, and then baked in the air at 250°C, 350°C, 400°C, and 450°C after drying. The gold catalysts supported by sintered Ni fibers were obtained, respectively denoted as Au-3-250 / X-Ni-8, Au-3-350 / X-Ni-8, Au-3-400 / X-Ni-8, Au -3-450 / X-Ni-8.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com