Method for synthesizing BTX aromatic methyl into unsym-trimethyl benzene
A technology for BTX aromatics and trimethylene, which is applied in the fields of condensation between hydrocarbons and non-hydrocarbons to produce hydrocarbons, organic chemistry, and isomerization to hydrocarbons, etc. It can solve the problems of no industrial application prospect and low yield of trimethylene. , to achieve good industrial application prospects, low cost of raw materials, and high selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of molecular sieve catalyst:
[0025]Separate preparation of SiO by hydrothermal synthesis 2 / Al 2 o 3 =55 NaZSM-5, SiO 2 / Al 2 o 3 =30 NaZSM-22, SiO 2 / Al 2 o 3 =50 NaMCM-22, SiO 2 / Al 2 o 3 =25 NaMCM-49, SiO 2 / Al 2 o 3 =25 NaMCM-56 molecular sieve and SiO 2 / Al 2 o 3 =30 Naβ molecular sieve, the resulting molecular sieve is exchanged with ammonium chloride, washed and roasted, then pressed into tablets and sieved to get a certain number of particles to obtain hydrogen molecular sieve catalysts HZSM-5, HZSM-22, HMCM-22, HMCM -49, HMCM-49, HMCM-56 and Hβ are used as catalysts for the synthesis of trimethylene by alkylation.
Embodiment 2~7
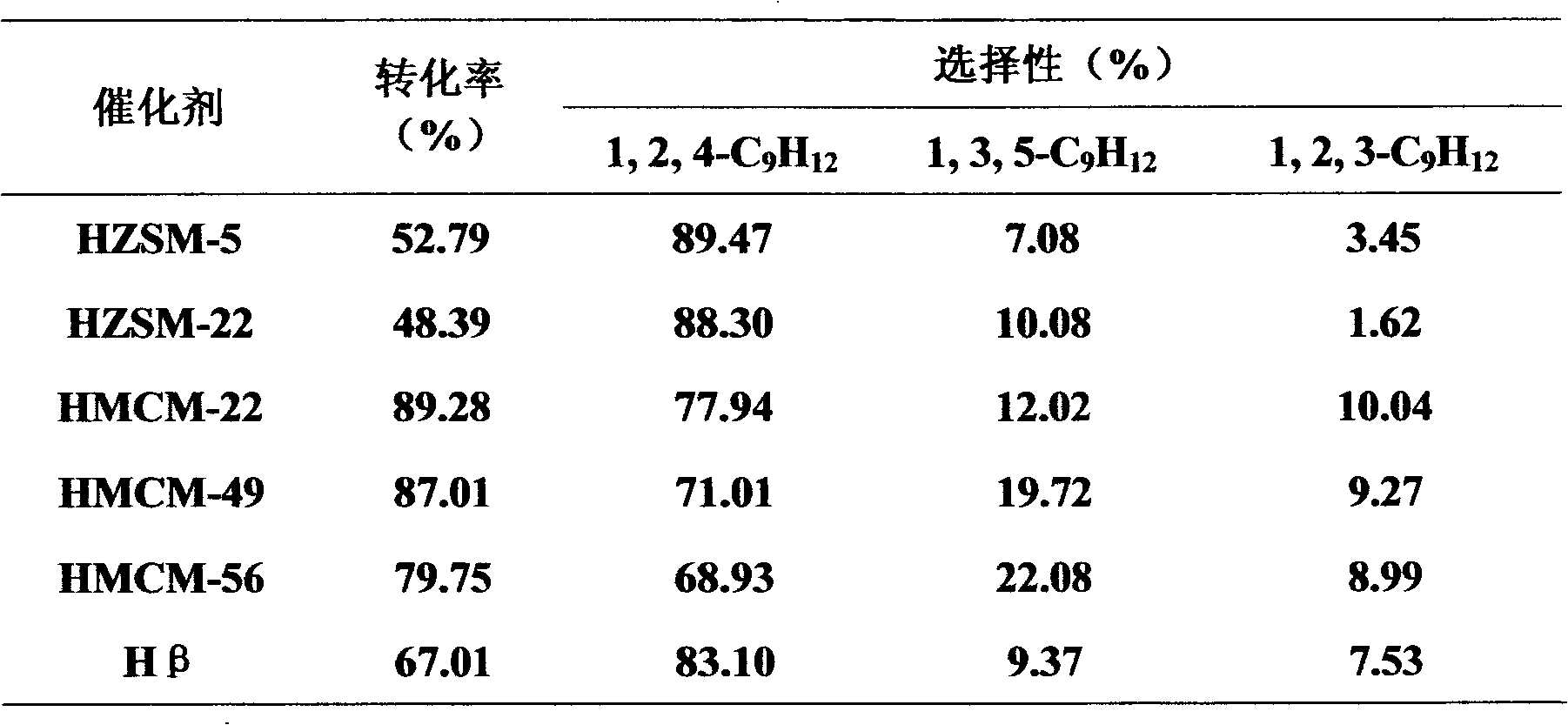
[0027] The catalyst prepared in Example 1 was applied to the alkylation reaction of toluene and methanol to synthesize trimitylene, and the fixed-bed continuous reaction was adopted. The reaction conditions are: temperature 350°C, liquid mass space velocity 1h -1 , Benzene: Methanol (molar ratio) 1:2, the reaction time is 1 hour, normal pressure. The reaction results are shown in Table 2.
[0028] Table 2 Reaction performance of toluene and methanol alkylation to synthesize mesitylene on different catalysts
[0029]
Embodiment 8~10
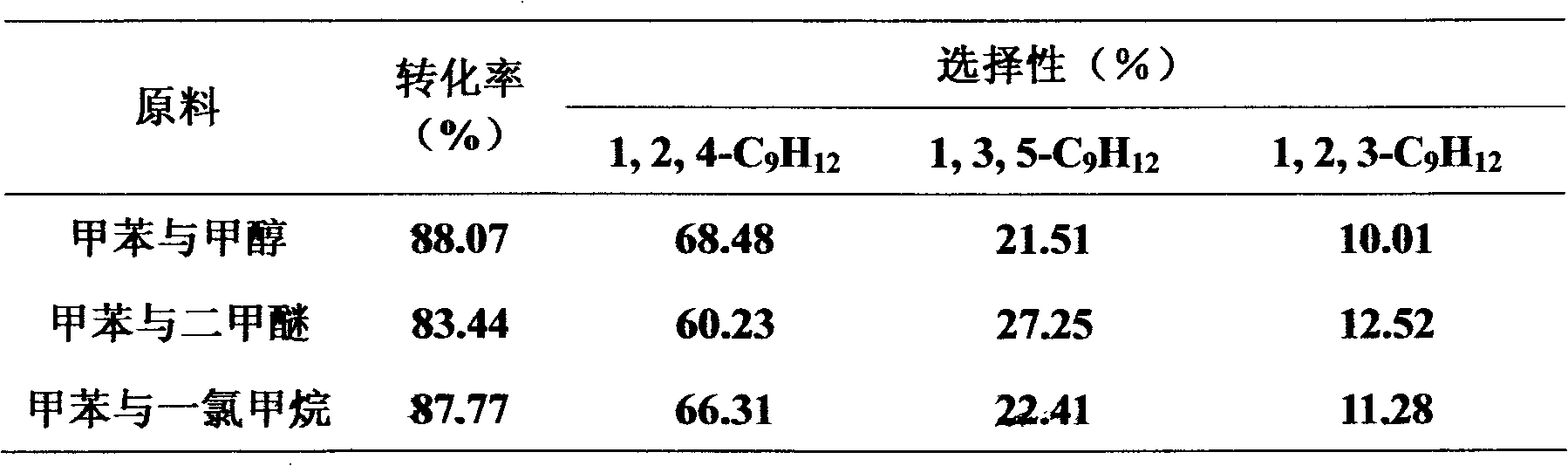
[0031] Toluene is alkylated with methanol, dimethyl ether or methylene chloride to synthesize trimitylene, and the fixed-bed continuous reaction is adopted.
[0032] Reaction conditions: temperature 400°C, liquid mass space velocity 1h -1 , toluene: methanol or methyl chloride (molar ratio) 1:2, toluene: dimethyl ether (molar ratio) 1:1, the reaction time is 1 hour, normal pressure. The catalyst is SiO 2 / Al 2 o 3 =50 HMCM-22 molecular sieve. The reaction results are shown in Table 3.
[0033] Table 3 Reaction performance of toluene alkylation to synthesize mesitylene on HMCM-22 catalyst
[0034]
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com