Anticoagulant compound, composition and application thereof
A compound and composition technology, applied in the field of new coumarin derivatives, can solve the problems of fast dissociation speed, unsatisfactory bioavailability, short half-life and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
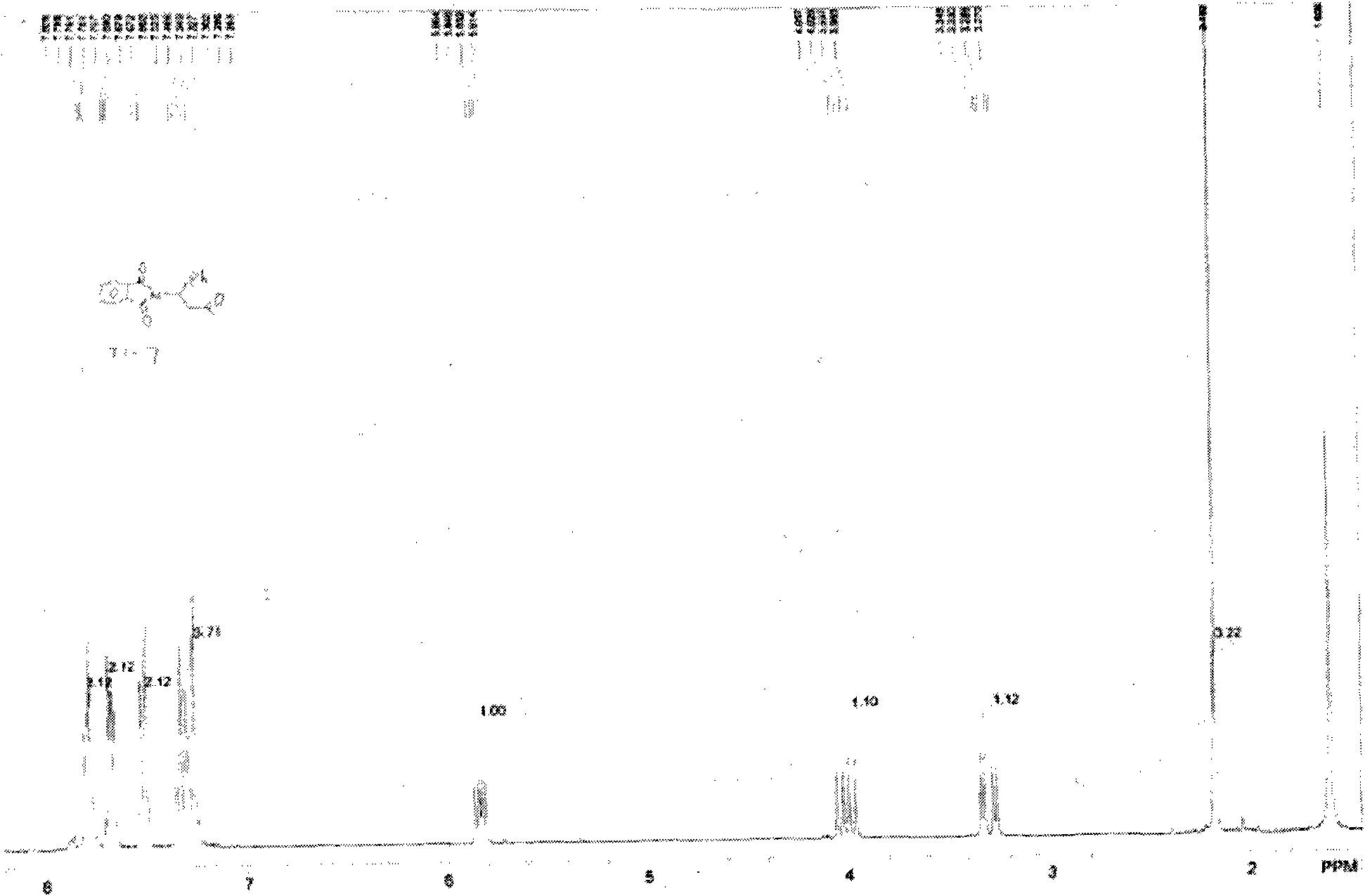
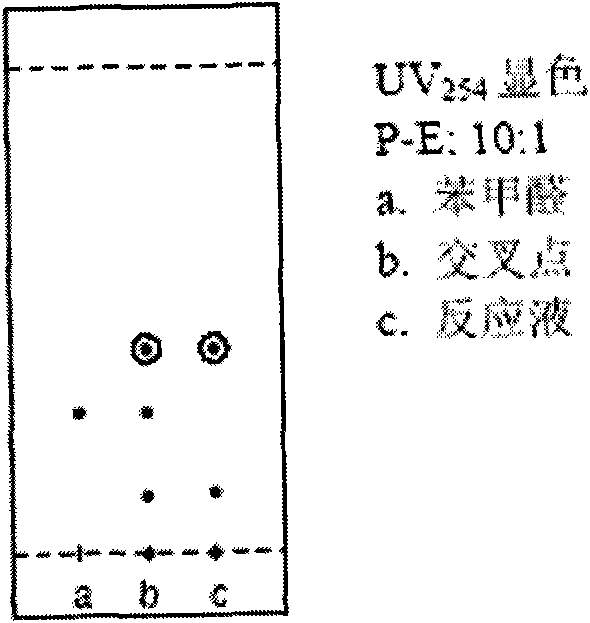
[0078] 1. Preparation of 4-phenyl-3-butene-2-one (7)
[0079]
[0080] Benzaldehyde (6)
[0081] Dissolve 120g sodium hydroxide in 1.08L distilled water to make a 10% sodium hydroxide aqueous solution, cool to 5℃ in an ice bath, add 30.5ml benzaldehyde and 88ml acetone, stir at 5-10℃ for 4 hours, thin layer chromatography Show that the reaction is complete. The reaction solution was extracted three times with dichloromethane, and the organic layers were combined, then washed with saturated ammonium chloride aqueous solution until neutral, and dried with anhydrous sodium sulfate overnight. After filtration, the filtrate was concentrated under reduced pressure to obtain a yellow oil. The crude product was distilled under reduced pressure, and the fraction at 106-108°C (under 3mmHg) was collected and placed in the refrigerator for a while to form a solid. Finally, 32 g of a light yellow solid (compound (7)) was obtained, yield: 74 %.
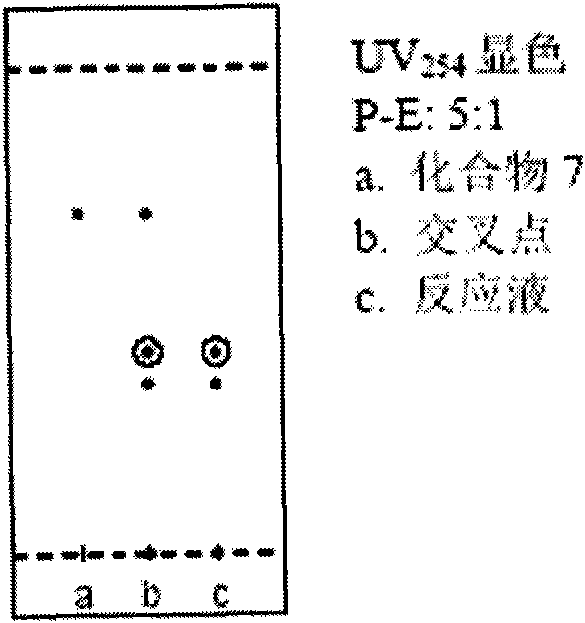
[0082] 2. Preparation of compound (2)
[008...
Embodiment 2
[0087] Take SD rats into random groups, 10 in each group, half male and half male. Set up a negative control group. Warfarin sodium is the positive control drug. 37 hours before the experiment, the rats were given by intragastric administration, the tails of the rats were cut off and blood was collected, and the clotting time was measured.
[0088] The test drug was dissolved in 0.5% CMC solution, the dosage of compound (2) obtained in Example 1 was 1 mg / kg, 3 mg / kg, 10 mg / kg, 30 mg / kg, 100 mg / kg; the dosage of the positive control drug was 1 mg / kg. kg, 3mg / kg, 10mg / kg, 30mg / kg, gavage volume is 10ml / kg, single gavage administration. The negative control group was given an equal volume of solvent.
[0089] Using clotting time as an indicator, a t test was performed to compare the significance of differences between groups.
[0090] Table 1 shows the results of rat coagulation time of compound (2) obtained in the examples.
[0091] The results of the rat clotting time of the positi...
Embodiment 3
[0100] Take SD rats into random groups, 4 in each group, half male and half male. Set up a negative control group. Warfarin sodium is the positive control drug. 37 hours before the experiment, the rats were given by intragastric administration, the tails of the rats were cut off and blood was collected, and the clotting time was measured.
[0101] The test drug was dissolved in 0.5% CMC solution, the dosage of compound (2) obtained in Example 1 and the positive control drug were both 10 mg / kg, the gavage volume was 10 ml / kg, and single gavage was administered. The negative control group was given an equal volume of solvent.
[0102] Using clotting time as an indicator, a t test was performed to compare the significance of differences between groups.
[0103] See Table 3 for the results of rat clotting time of the tested drug.
[0104] Table 3 Experimental results of coagulation time of rat tail docking
[0105]
[0106] *p<0.05 **p<0.01 Comparison between each group and the negative...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com