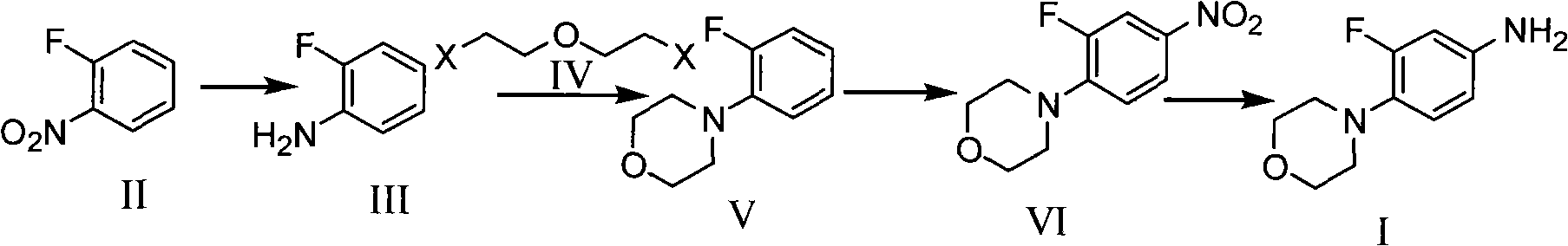
Method for preparing 3-fluorine-4 morpholinyl phenylamine
A technology of morpholinoaniline and morpholinobenzene, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of complex post-treatment of fluorine-containing wastewater and high price, and achieve the effects of low price, less environmental pollution, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Add 100ml of water, 1.44g (0.04mol) of hydrochloric acid with a mass fraction of 36% and 22.3g (0.4mol) of iron powder in a 250ml reaction bottle equipped with a dropping and reflux device, slowly add o-fluorine dropwise under reflux Add 14.1g (0.1mol) of nitrobenzene, reflux for 2 hours after addition, cool at 50°C, add 100ml of toluene, filter, wash the filter cake once with 20ml of toluene, and discard the iron sludge. The layers were separated, and the aqueous layer was extracted once with 50 ml of toluene. The toluene layers were combined, concentrated under reduced pressure, and rectified to obtain 10 g of o-fluoroaniline, with a yield of 90%.
[0029] (2) Dissolve 66g (0.6mol) of o-fluoroaniline obtained in step (1) in 300ml of ethylene glycol, then add 99g (0.92mol) of anhydrous sodium carbonate, and dropwise add 127.5g (0.9mol) of di Chloroethyl ether, dropwise, stirred at 150°C for 5 hours to complete the reaction, cooled at 50°C, extracted with 2×100ml to...
Embodiment 2
[0033] (1) Add 20g (0.142mol) o-fluoronitrobenzene, 130ml methanol, and 1g Raney nickel into a 200ml autoclave, adjust the hydrogen pressure to 20 atmospheres, and stir at room temperature for 10 hours to complete the reaction. The reaction solution was obtained by filtration, and the filtrate was concentrated under reduced pressure and rectified to obtain 14.2 g of an oily substance. The yield was 90%.
[0034] (2) According to the method for preparing o-fluoro-morpholinylbenzene in step (2) of Example 1, ethylene glycol is replaced with diethylene glycol dimethyl ether solvent to obtain 86g of o-fluoro-morpholinylbenzene with a yield of 80 %.
[0035] (3) According to the method for preparing o-fluoro-morpholinyl nitrobenzene according to step (3) of Example 1, the concentrated nitric acid with a mass fraction of 68% was replaced with fuming nitric acid with a mass fraction of 98%, to obtain 57.7g of solid, with a yield of 85% .
[0036] (4) Add 50 g (0.22 mol) of 3-fluor...
Embodiment 3
[0038] (1) According to the catalytic hydrogenation reduction method of the second step (1) of the embodiment, the Raney nickel is replaced with palladium carbon.
[0039] (2) According to the method for preparing o-fluoro-morpholinylbenzene in step (2) of Example 1, anhydrous sodium carbonate was replaced with anhydrous potassium carbonate to obtain 80 g of o-fluoro-morpholinylbenzene with a yield of 74.3%.
[0040] (3) Same as embodiment one step (3).
[0041] (4) With embodiment two steps (4).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com