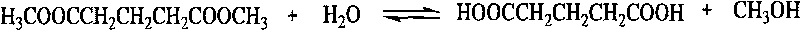Method for preparing glutaric acid from dimethyl glutarate
A technology of dimethyl glutarate and glutaric acid, which is applied in the preparation of carboxylate/lactone, chemical instruments and methods, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of incomplete process , There are no problems such as public technology, complex process route, etc., to achieve the effect of improving catalytic yield and reaction rate, convenient source and simple reaction equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 150mL single-necked round-bottom flask, add the activated strong acid styrene-based cation exchange resin catalyst, dimethyl glutarate and deionized water to form a mixed system, so that the amount of the catalyst in the mixed system is 170g / L, pentadiene The volume ratio of dimethyl ester to deionized water is 12:1, a fractionation device is connected above the flask, heated to 130°C, and the reaction time is 2h.
[0029] The glutaric acid yield was measured to be 88.84%.
Embodiment 2
[0031] As in Example 1, but the amount of catalyst is 130g / L. The glutaric acid yield was measured to be 87.14%.
Embodiment 3
[0033] As in Example 1, but the amount of catalyst is 200g / L. The glutaric acid yield was measured to be 88.24%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com