Polybenzimidazole material and midbody organic diacid thereof as well as preparation methods thereof
A benzimidazole and organic technology, which is applied in the field of functional polymer material preparation, can solve the problems of oxidative degradation of membranes and needs to be further improved, and achieves the effects of easy availability of raw materials, suitable for large-scale production, and improved solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
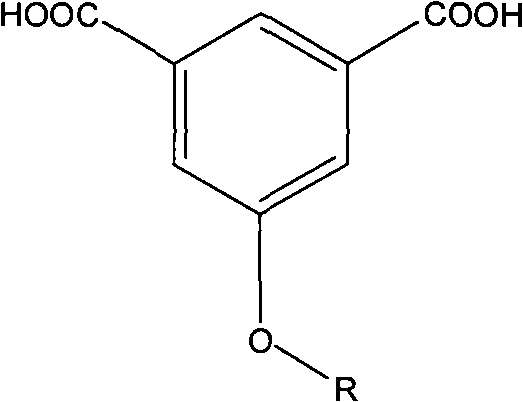
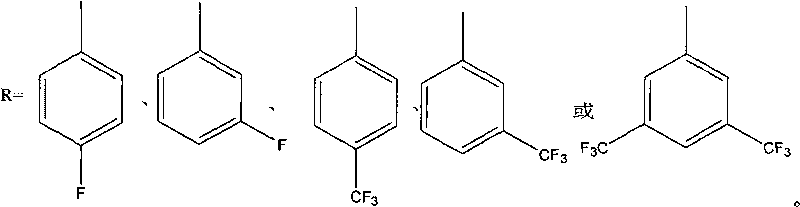
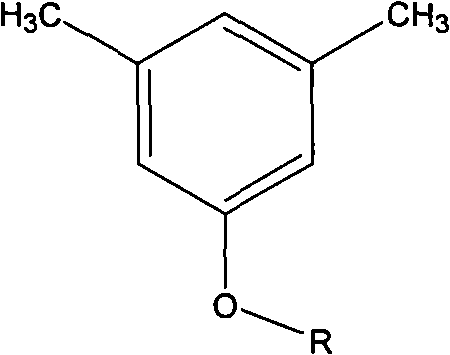
[0048] in N 2 Add 175 parts of p-fluorobromobenzene, 122 parts of 3,5-dimethylphenol, 80 parts of Na2CO3, 800 parts of dimethylformamide and 200 parts of toluene into the three-necked flask under protection, start stirring, and slowly heat to reflux for 5 hours , exclude toluene, heat up to 130 ° C for 10 h, pour the reaction mixture into ethanol / water solution, filter and collect the filtrate and extract it with ether for 3 times, collect the oil phase and concentrate, vacuum distill to obtain 3,5-dimethyl-(4 -fluoro-phenoxy)-benzene. Add 100 parts of 5-(4-fluoro-phenoxy)-1,3-dimethylbenzene, 500 parts of water, and 500 parts of pyridine into a 2L three-necked flask, heat to reflux, slowly add potassium permanganate in portions 100 parts, reflux reaction for 7 days, pour the reactant into excess 10% hydrochloric acid, collect the precipitate by filtration, recrystallize the precipitate with ethanol and dry it in vacuum to obtain 5-(4-fluoro-phenoxy)-resorcinol formic acid. ...
Embodiment 2
[0051] in N 2 Add 225 parts of 4-trifluoromethylbromobenzene, 122 parts of 3,5-dimethylphenol, 80 parts of K2CO3, 1000 parts of dimethylacetamide and 300 parts of toluene into the three-necked flask under protection, start stirring, and heat slowly To reflux for 6 hours, remove toluene, raise the temperature to 140°C for 10 hours, pour the reaction mixture into ethanol / water solution, collect the filtrate by filtration and extract it with ether for 3 times, collect the oil phase and concentrate, then vacuum distill to obtain 3,5-dimethyl yl-(4-trifluoromethyl-phenoxy)-benzene. Add 100 parts of 3,5-dimethyl-(4-trifluoromethyl-phenoxy)-benzene and 500 parts of 50% nitric acid into a 1L autoclave lined with tetrafluoroethylene, and react at 150°C and 5 atm for 3 Hours, after cooling and reducing pressure, the reaction mixture was taken out and the precipitate was collected by filtration. The precipitate was recrystallized with ethanol / water and dried in vacuo to obtain 5-(4-trif...
Embodiment 3
[0054] Add 250 parts of 3,5-trifluoromethylchlorobenzene, 122 parts of 3,5-dimethylphenol, 100 parts of KOH, 1000 parts of dimethyl sulfoxide and 300 parts of toluene into the three-necked flask under the protection of argon, Start stirring, slowly heat to reflux for 5 hours, remove toluene, raise the temperature to 130°C for 12 hours, pour the reaction mixture into ethanol / water solution, collect the filtrate by filtration and extract with chloroform for 3 times, collect the oil phase and concentrate, then vacuum distill to obtain 3 , 5-Dimethyl-(3,5-trifluoromethyl-phenoxy)-benzene. Add 150 parts of 5-(4-fluoro-phenoxy)-1,3-dimethylbenzene, 600 parts of water, and 600 parts of pyridine into a 2L three-necked flask, heat to reflux, slowly add potassium permanganate in portions 120 parts, reflux reaction for 6 days, the reactant was poured into excess 10% hydrochloric acid, the precipitate was collected by filtration, and the precipitate was recrystallized with ethanol / water a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com