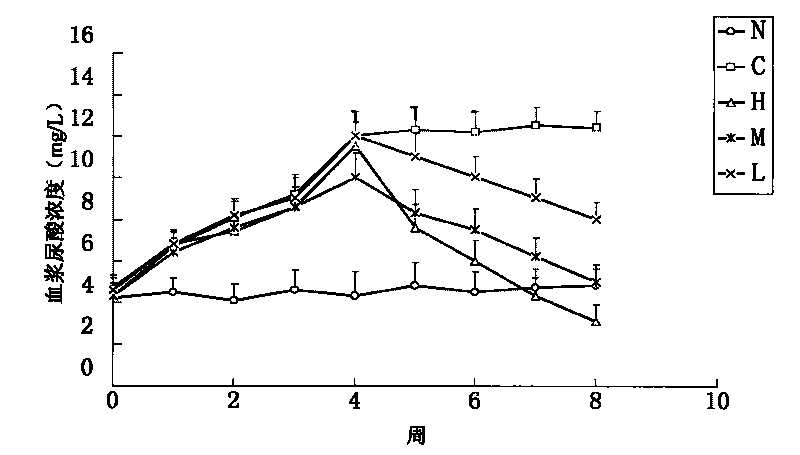
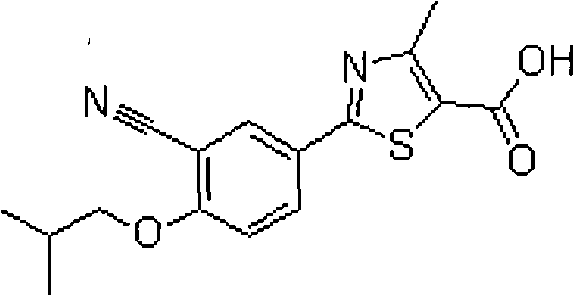
Febuxostat enteric preparation
A febuxostat preparation technology, applied in the field of enteric-coated preparations of febuxostat, can solve the problems of gastrointestinal irritation, gastric ulcer, easy damage to gastric mucosa, etc., and achieve the effect of fast dissolution and absorption and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Enteric-coated Febuxostat Tablets
[0022] (1) chip core
[0023] Prescription 1000 tablets
[0024] Febuxostat 80g
[0025] 20g pregelatinized starch
[0026] Hydroxypropyl Cellulose 10g
[0027] Preparation: Take febuxostat, pregelatinized starch, and hydroxypropyl cellulose and pulverize them into an 80-mesh sieve respectively, weigh and mix according to the prescription amount, and use 2% ethanol aqueous solution of hydroxypropyl methyl cellulose (80% ) appropriate amount, the soft material is passed through a 16-mesh sieve, granulated, dried at 50-60°C, and granulated with a 18-mesh sieve. Measure the content of granules, press into tablets, and obtain. Each tablet weighs 120mg and contains 80mg of febuxostat.
[0028] (2) isolation layer
[0029] prescription
[0030] Hypromellose Phthalate 2g
[0031] Tween-80 2g
[0032] Ethanol 80ml
[0033] water 20ml
[0034] Dosing: Take the prescribed amount of hypromellose phthalate and disperse it in...
Embodiment 2
[0043] Example 2 Enteric-coated febuxostat pellets
[0044] (1) Prescription of pellets
[0045] Febuxostat 80g
[0046] Lactose 250g
[0047] Silicified Microcrystalline Cellulose 50g
[0048] Preparation: take three kinds of raw materials and auxiliary materials, and prepare it with 2% polyvinylpyrrolidone aqueous solution, and the diameter is 0.5-0.6mm.
[0049] (2) Package isolation layer
[0050] prescription
[0051] Hydroxypropyl Methyl Cellulose Phthalate 5g
[0052] Tween-80 3g
[0053] Ethanol 80ml
[0054] water 20ml
[0055] Preparation and coating are the same as in Example 1, the rotating speed of the coating pan is adjusted to 50 rpm, the air temperature is controlled at about 40°C, the gap is sprayed with an isolation layer, the weight of the pellets is increased by 2%, and it is taken out and dried at 40°C.
[0056] (3) Enteric coating
[0057] prescription
[0058] Polyvinyl acetate phthalate 12g
[0059] Diethyl phthalate 2g
[0060] Stearic acid ...
Embodiment 3
[0063] Example 3 Enteric-coated Febuxostat Tablets
[0064] (1) chip core
[0065] Prescription 1000 tablets
[0066] Febuxostat 120g
[0067] 40g pregelatinized starch
[0068] Hydroxypropyl Cellulose 20g
[0069] Preparation: Same as Example 1, each tablet weighs 180 mg, containing 120 mg of febuxostat.
[0070] (2) isolation layer
[0071] prescription
[0072] Hypromellose Phthalate 10g
[0073] Tween-80 5g
[0074] Ethanol 80ml
[0075] water 20ml
[0076] Dosing: with embodiment 1
[0077] Preparation: with embodiment 1
[0078] Weight gain 4mg per tablet
[0079] (3) Enteric coating
[0080] prescription
[0081] Cellulose acetate 10g
[0082] Phthalic acid 6g
[0083] Ethanol 80ml
[0084] water 20ml
[0085] The preparation and coating are the same as the isolation layer, each tablet has a weight gain of 18 mg, and then taken out and dried overnight at 40°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com