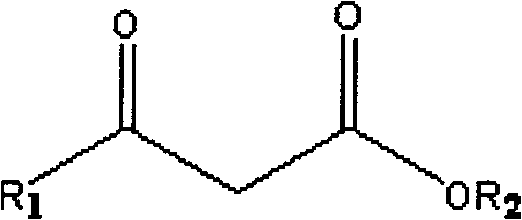
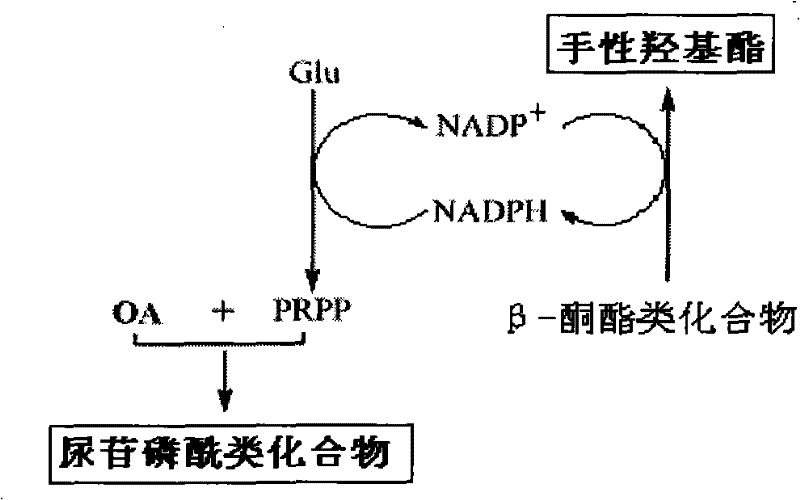
A kind of method that uridine phosphoryl compound co-produces chiral hydroxyl ester
A technology of uridine phosphoryl and compound is applied in the field of preparation of biochemical products, can solve the problems of low yield and high cost, achieve the effects of high conversion efficiency, low cost, and overcome low substrate conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Uridine-phosphate (UMP) co-production of (S)-(-)-4-chloro-3-hydroxybutyric acid ethyl ester.
[0040] In a reaction tank with a capacity of 15L, 10 grams of orotic acid OA, 50 grams of ethyl 4-chloroacetoacetate, 500 grams of glucose, 500 grams of commercially available baker's yeast powder, magnesium sulfate 10mM, potassium dihydrogen phosphate 0.01M, 10 L of reaction solution consisting of 12 ml of xylene and water was used to adjust the pH to 8.5 with sodium hydroxide, and the reaction was stirred at 30° C. for 20 h at high speed, and the ventilation rate was 0.5 (V / V·min). After the reaction, inactivate with perchloric acid, extract (S)-(-)-4-chloro-3 hydroxybutyric acid ethyl ester with acetoacetic acid, use HPLC and GC to analyze uridine monophosphate (UMP) and (S )-(-)-4-chloro-3-hydroxybutyric acid ethyl ester is quantitatively analyzed, and the optical rotation value of product (S)-(-)-4-chloro-3-hydroxybutyric acid ethyl ester is measured with a pol...
Embodiment 2
[0041] Example 2: Coproduction of uridine diphosphate D-glucose (UDP-Glc) (S)-(+)-4,4,4-trifluoro-3-hydroxybutyric acid ethyl ester ((S)-TFHBE) .
[0042] Yeast medium (g / L): glucose 40, urea 2.0, potassium dihydrogen phosphate 1.5, magnesium sulfate heptahydrate 0.5, zinc sulfate heptahydrate 4.0×10 -3 , ferrous sulfate heptahydrate 3.0×10 -3 , manganese chloride tetrahydrate 0.3×10 -3 , anhydrous calcium chloride 1.0×10 -3 , Biotin 0.05×10 -3 . Saccharomyces cerevisiae inoculum was 10% (v / v), cultured on a shaker at 120 rpm at 30°C for 24 hours, and centrifuged at 4000 rpm for 20 minutes. Take the yeast paste and store it at -7°C for later use.
[0043] In a reaction tank with a capacity of 15L, 100 grams of OA, 3000 grams of glucose, 500 grams of 4,4,4-trifluoro-acetoacetate (TFAAE), 5000 grams of Saccharomyces cerevisiae mud, air-dried treatment, magnesium sulfate 200mM, Sodium dihydrogen phosphate 2M, Triton X-100 10ml and 10L of reaction solution composed of water...
Embodiment 3
[0044] Example 3: Coproduction of ethyl (S)-(-)-4-bromo-3-hydroxybutyrate from uridine triphosphate (UTP).
[0045] In the reaction tank with a capacity of 15L, 60 grams of OA, 1400 grams of glucose, 200 grams of ethyl 4-bromoacetoacetate, 20 mM of magnesium sulfate, and 2700 grams of baker's yeast were cultivated by the method described in Example 2, and freeze-thawed 3 times. Disodium hydrogen phosphate 0.6M, cetyl trimethylamine ammonium bromide 10 g and water reaction solution 10L, adjust the pH to 7 with sodium hydroxide, the ventilation rate is 1.0 (V / V min), in 30 Stir the reaction at high speed for 16 hours at ℃, then stop the ventilation, reduce the stirring rate, raise the temperature to 37℃, adjust the pH to 6.5 with HCl, add 500 grams of glucose, and react for another 2 hours. After the reaction, inactivate with perchloric acid , extracted (S)-(-)-4-bromo-3-hydroxybutyrate ethyl ester with ethyl acetate, and used HPLC and GC to analyze uridine triphosphate (UTP) an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com