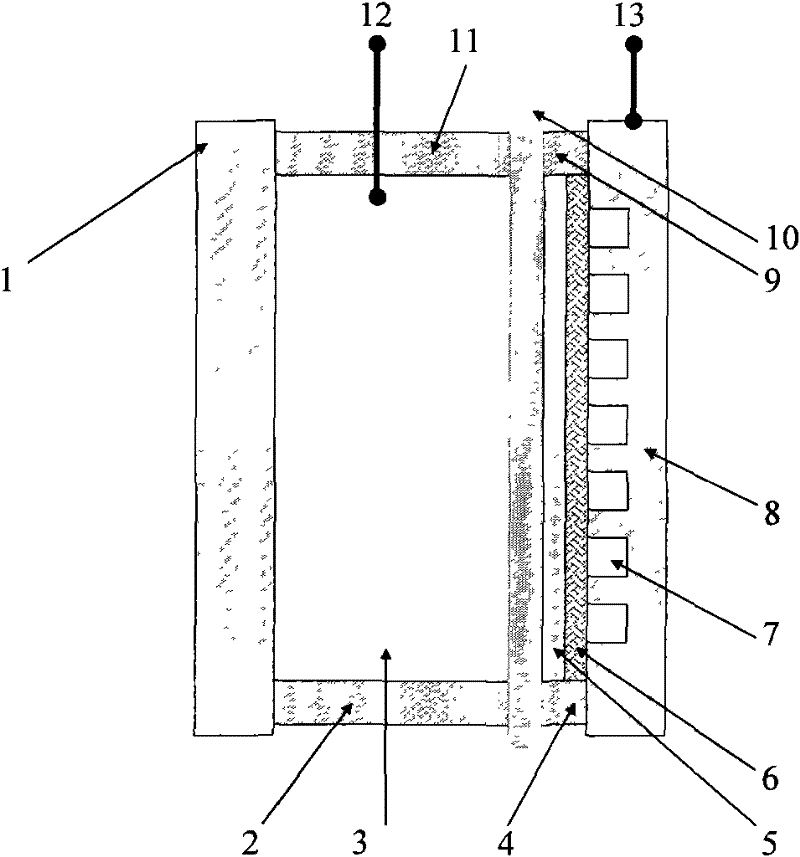
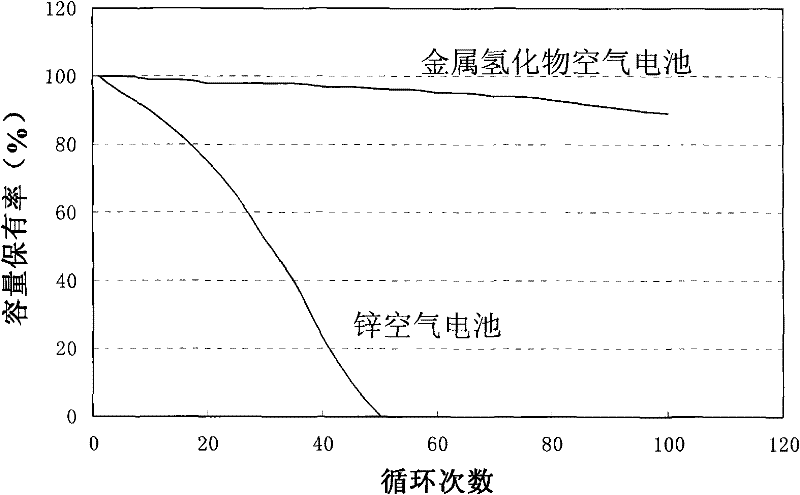
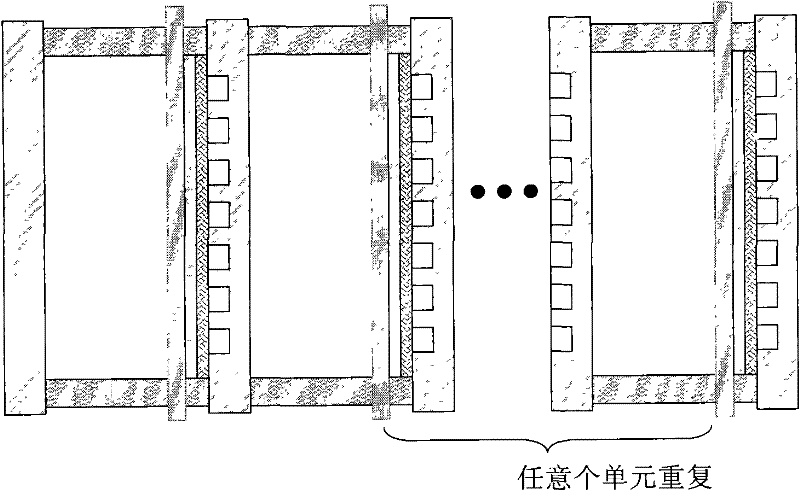
Rechargeable metal hydride air battery
A technology of air batteries and hydrides, applied in electrical components, battery electrodes, circuits, etc., can solve problems such as diaphragm penetration and battery failure, and achieve the effects of increasing the number of times of use, improving life, and high social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment one: with LaNi 5 Rechargeable air battery as anode material
[0055] Sieve LaNi with particle size less than 10 microns 5 fine powder, LaNi 5 Fine powder and sodium carboxymethyl cellulose solution (5wt.%) as a binder are prepared into a slurry in a mass ratio of 1:1, coated into foamed nickel, dried at room temperature, and pressed into shape to obtain LaNi 5 The negative terminal of the air battery. LaNi 5 The mass ratio to nickel foam is 1:0.5.
[0056] Disperse carbon black in water to form a suspension with a mass ratio of 1:15; add glacial acetic acid to adjust the pH value to 2.5, and stir at room temperature for 30 minutes; the mass ratio of carbon black and pyrrole is 1:0.3, add pyrrole and stir for 5 minutes, Then add cobaltous chloride as the initiator of polymerization, the mass ratio of carbon black and cobaltous chloride is 1: 4, then add 10 milliliters of hydrogen peroxide (concentration is 0.5wt.%) to accelerate polymerization speed, afte...
Embodiment 2
[0058] Embodiment two: with MmNi 3.9 V 0.1 Air battery with Al as negative electrode material
[0059] MmNi with a particle size greater than 10 microns and less than 100 microns 3.9 V 0.1 Al fine powder and polyvinyl alcohol (PVA) solution (5wt.%) were mixed at a mass ratio of 1:2 to form a slurry coated on copper foam, dried at room temperature and then pressed into shape to obtain a negative electrode for an air battery. MmNi 3.9 V 0.1 The mass ratio of Al to copper foam is 1:10.
[0060] Disperse carbon black in methanol to form a suspension with a mass ratio of 1:15, add hydrochloric acid to adjust the pH value to 3, and stir at room temperature for 20 minutes; the mass ratio of carbon black and pyrrole is 1:0.05, add pyrrole and stir for 10 minutes, Then add vanadium chloride as the initiator of the polymerization reaction, the mass ratio of carbon black and vanadium chloride is 1: 5, then add 15 milliliters of hydrogen peroxide (concentration is 0.5wt.%) to accele...
Embodiment 3
[0062] Embodiment three: with MlNi 4.5 co 0.25 Al 0.25 Air battery as negative electrode material
[0063] MlNi 4.5 co 0.25 Al 0.25 Fine powder (100 microns) and PVA aqueous solution (5wt.%) are mixed in a mass ratio of 1:3 to form a slurry, which is applied to a carbon fiber mat, dried at room temperature, and pressed into shape to obtain a negative electrode for an air battery, MlNi 4.5 co 0.25 Al 0.25 The mass ratio to carbon fiber felt is 1:0.05.
[0064]Disperse carbon black in chloroform to form a suspension, the mass ratio of which is 1:15, add hydrochloric acid to adjust the pH value to 3, and stir at room temperature for 10 min; the mass ratio of carbon black and pyrrole is 1:0.1, add pyrrole and stir for 5 min, Then add manganese chloride, cobalt chloride as the initiator of polymerization, the mass ratio of carbon black and manganese chloride, cobalt chloride is 1: 2.5: 2.5, then add hydrogen peroxide (concentration is 0.5wt.%) 20 milliliters or more Accele...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com