Method for electrolyzing aluminum from ionic liquid on hyper-gravity basis
An ionic liquid and electrolytic aluminum technology, which is applied in the field of metal electrodeposition, can solve the problems of difficult to achieve good electroplating of metal aluminum, it takes a long time, and the production efficiency is low, so as to shorten the preparation cycle, solve the problem of easy falling off, and avoid dendrites The effect of aluminum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
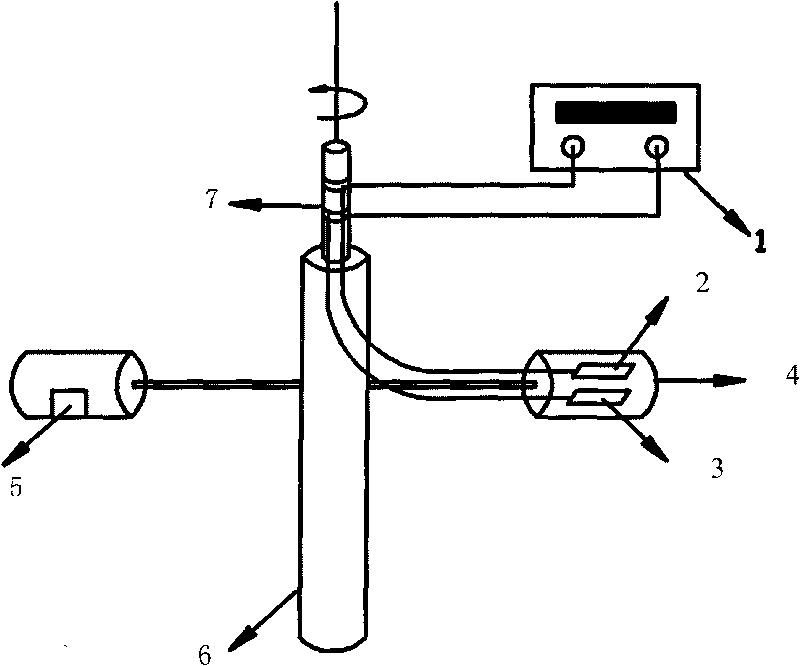
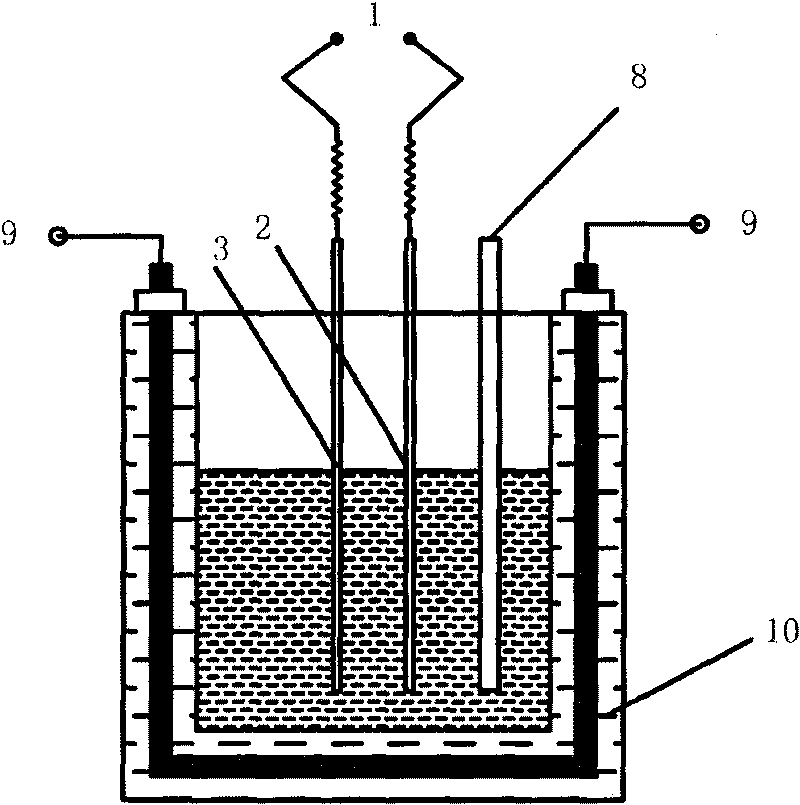
[0023] Under nitrogen protection, prepare AlCl 3 -BMIC (1-butyl-3-methylimidazolium chlorate) ionic liquid, AlCl 3 The molar ratio to BMIC is 1.5-2.5:1. The ionic liquid is used as the electrolyte, the stainless steel sheet is used as the cathode, and the soluble aluminum-based composite material is used as the anode. The two electrodes are placed parallel to the direction of the centrifugal force, and the distance between the electrodes is fixed. Set a certain temperature, current density and cell voltage during the electrolysis process. After the electrolysis, the cathode sheet was soaked in absolute ethanol, and after drying, the aluminum layer was peeled off and weighed, and the deposition rate and current efficiency of the obtained aluminum were calculated.
[0024] Prepare AlCl with molar ratios of 1.5:1, 2:1 and 2.5:1 respectively 3 -BMIC ionic liquid, the experimental conditions and conclusions are shown in the following table:
[0025]
Embodiment 2
[0027] Under nitrogen protection, prepare AlCl 3 - EMIC (1-ethyl-3-methylimidazolium chlorate) ionic liquid, AlCl 3 The molar ratio to EMIC is 1.5-2.5:1. The ionic liquid is used as the electrolyte, the stainless steel sheet is used as the cathode, and the soluble aluminum-based composite material is used as the anode. The two electrodes are placed parallel to the direction of the centrifugal force, and the distance between the electrodes is fixed. Set a certain temperature, current density and cell voltage during the electrolysis process. After the electrolysis, the cathode sheet was soaked in absolute ethanol, and after drying, the aluminum layer was peeled off and weighed, and the deposition rate and current efficiency of the obtained aluminum were calculated.
[0028] Prepare AlCl with molar ratios of 1.5:1, 2:1 and 2.5:1 respectively 3 -EMIC ionic liquid, the experimental conditions and conclusions are shown in the table below:
[0029]
Embodiment 3
[0031] Under argon protection, prepare AlCl 3 -BPC (n-butylpyridinium chloride) ionic liquid, AlCl 3 The molar ratio with BPC is 1.5~2:1. The ionic liquid is used as the electrolyte, the stainless steel sheet is used as the cathode, and the soluble aluminum-based composite material is used as the anode. The two electrodes are placed parallel to the direction of the centrifugal force, and the distance between the electrodes is fixed. Set a certain temperature, current density and cell voltage during the electrolysis process. After the electrolysis, the cathode sheet was soaked in absolute ethanol, and after drying, the aluminum layer was peeled off and weighed. The calculated aluminum deposition rate and current efficiency are .
[0032] Prepare AlCl with molar ratios of 1.5:1, 1.75:1 and 2:1 respectively 3 -BPC ionic liquid, the experimental conditions and conclusions are shown in the table below:
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com