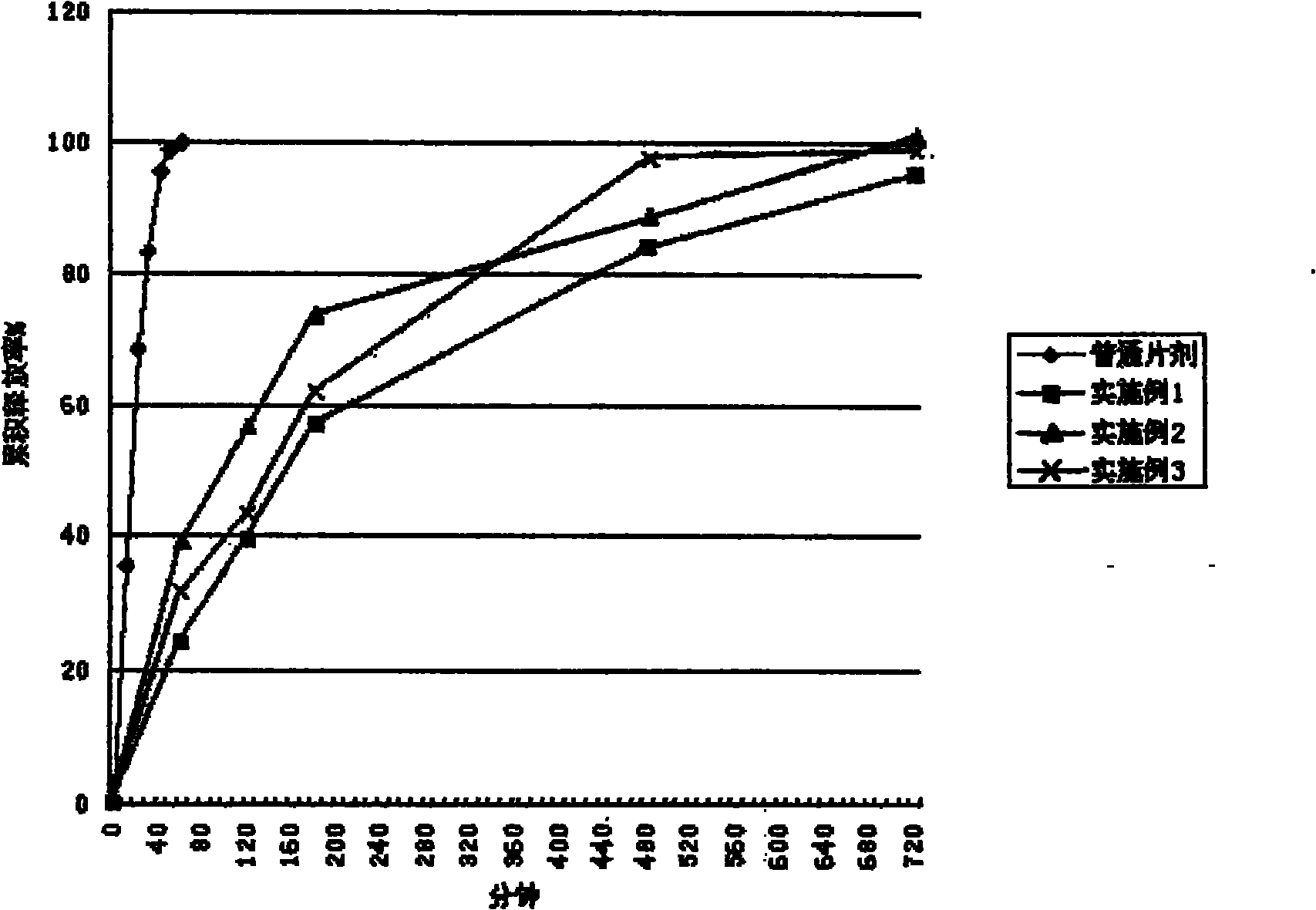
Slow-release preparation of cucurbitacin
A sustained-release preparation and technology of gentianin, which is applied to medical preparations containing active ingredients, organic active ingredients, and non-active ingredients of polymer compounds, etc., can solve problems such as inconvenient taking and inability to achieve drug release, and achieve peak reduction Valley phenomenon, reducing fluctuations, and stabilizing the effect of blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] composition:
[0027] Snow gallin 18g
[0028] Ethylcellulose 36g
[0029] Stearic acid 54g
[0030] Hydroxypropyl Cellulose 9g
[0031] Dextrin 22g
[0032] Micronized silica gel 3.2g
[0033] Magnesium stearate 0.9g
[0034] Preparation method: Take 54g of stearic acid, 6g of hydroxypropyl cellulose, and 18g of gentianin, pulverize them, pass through a 100-mesh sieve, disperse them evenly in ethyl acetate, and evaporate the solvent to dryness. Crush and pass through an 80-mesh sieve. Another 36g of ethyl cellulose and 3g of hydroxypropyl cellulose were mixed and pulverized, passed through an 80-mesh sieve, added 22g of dextrin passed through an 80-mesh sieve and medicinal powder containing gentianin, mixed evenly, and granulated with 95% ethanol , dried, and granulated through a 40-mesh sieve. Add 3.2 g of micropowder silica gel and 0.9 g of magnesium stearate, mix and press into tablets to make slow-release tablets each containing 18 mg of gentianin.
Embodiment 2
[0036] composition:
[0037] Sustained release portion Immediate release portion
[0038] Snow gallin 18g 6g
[0039] Hydroxypropyl Methyl Cellulose 50g
[0040] Macrogol 1600 20g
[0041] Triethanolamine 6g
[0043] Lactose 50g
[0044] Microcrystalline Cellulose 18g
[0045] Medicinal Calcium Carbonate 10g
[0046] Magnesium stearate 0.7g 0.5g
[0047] Preparation method:
[0048]1. Sustained-release part: Take 46g of hydroxypropyl methylcellulose, 20g of polyethylene glycol, 6g of triethanolamine, 5g of sodium stearate, and 18g of gentianin, mix them well, pulverize them, and pass through a 100-mesh sieve. Another 4 g of hydroxypropyl methylcellulose was prepared into a 3% solution with an appropriate amount of ethanol, granulated, dried, granulated with a 40-mesh sieve, added with 0.7 g of magnesium stearate, and mixed uniformly.
[0049] 2. The quick-release part: take 6g of gentianin, 30g of sodium stearate, ...
Embodiment 3
[0052] composition:
[0053] Sustained release portion Immediate release portion
[0054] Snow gallin 9g 3g
[0055] Methyl methacrylate 41.5g
[0056] Glyceryl monostearate 20g
[0057] Macrogol 400 7g
[0058] Hydroxypropyl Cellulose 2.2g
[0059] Micronized silica gel 22g
[0060] Magnesium stearate 0.5g 0.3g
[0061] Lactose 22g
[0062] Dextrin 10g
[0063] Starch 5.5g
[0064] Preparation method:
[0065] 1. Preparation of sustained-release pellets:
[0066] Get gentianin 9g, pulverize after mixing micropowder silica gel 22g, cross 100 mesh sieves. Glyceryl monostearate 20g, after heating and melting at 70-75°C, add 35g methyl methacrylate and 7g polyethylene glycol 400, mix well, and keep warm. Add pectin-containing medicinal powder, fully stir and mix, and gradually lower the temperature, adjust the stirring speed, when granules are formed, add 0.5g of magnesium stearate, continue to stir, and make round pellets with a particle size of 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com