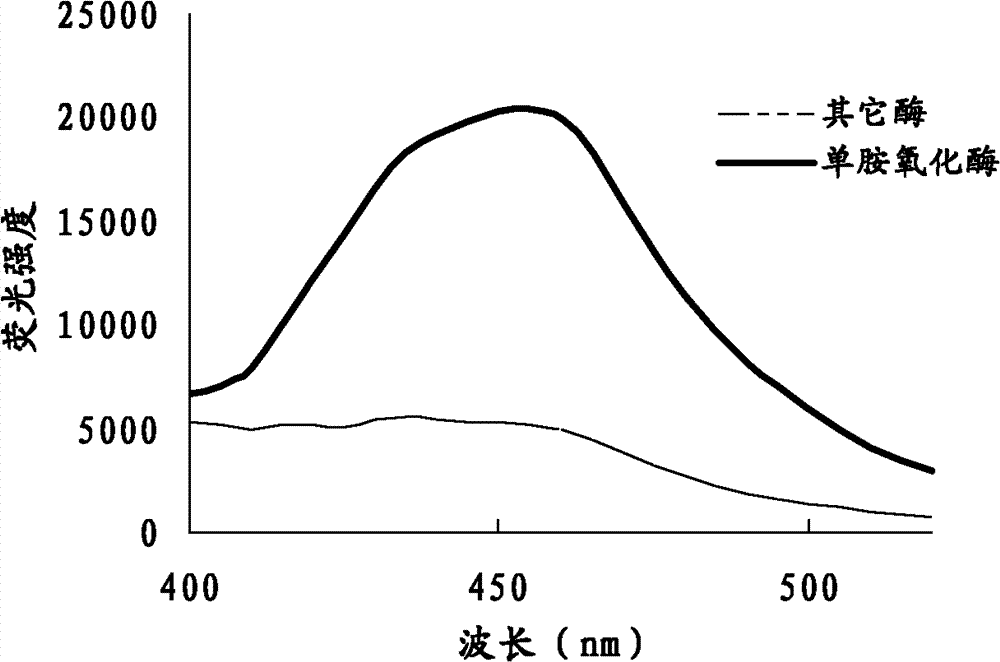
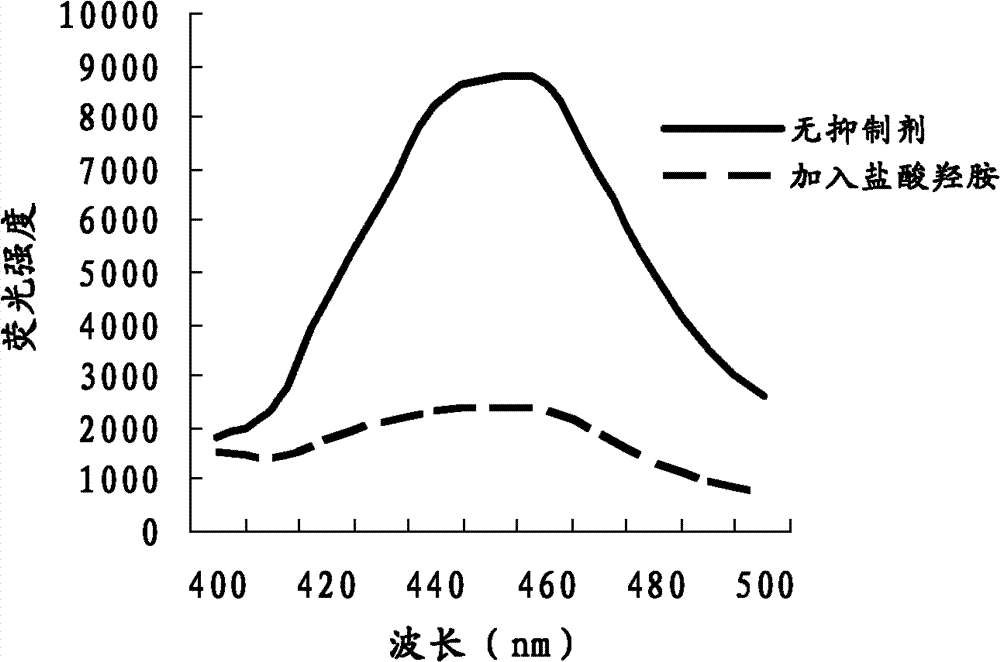
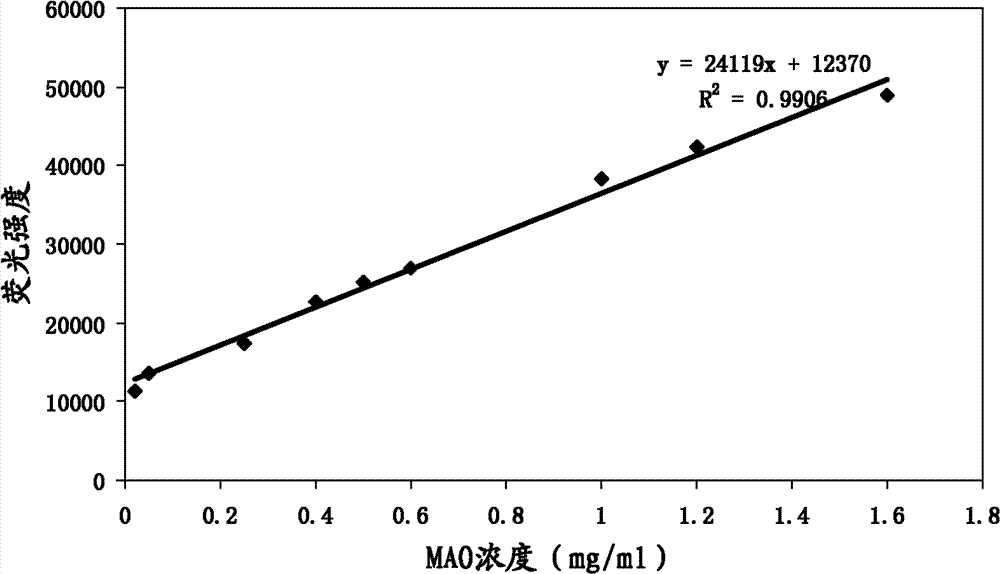
Compound and method applying same for fluorescence detection of activity of monoamine oxidase
A technology of monoamine oxidase and compound, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve problems such as complex preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, preparation 4-methyl-7-(1-methyl-1,2,3,6-tetrahydropyridine-4-oxyl group)-coumarin
[0037]
[0038] 0.1mmol (15.8mg) 4-bromopyridine was reacted with 0.6mmol (85.2mg) methyl iodide at 0°C for 12 hours, and 1-methyl-4-bromopyridinium salt was obtained after the reaction, and 1-methyl-4 -Bromopyridinium salt, 0.1mol (14.6mg) coumarin, 0.2mmol (10.8mg) sodium methoxide in 20ml DMF solvent, react at room temperature for 12 hours, filter the reaction solution after the reaction, take the filter cake at 0°C , carry out reduction reaction with 0.2mmol (7.2mg) sodium borohydride, add saturated NaCl aqueous solution to the reaction solution after the reaction, extract with ethyl acetate, take the organic layer and pass through drying, filtration, evaporate to dryness, obtain the crude product, the crude product carries out column layer Analysis, with the volume ratio of ethyl acetate and petroleum ether being 5:1 solution as the mobile phase, TLC tracking and c...
Embodiment 2
[0043] Example 2: Preparation of 4-allyl-7-(1-methyl-1,2,3,6-tetrahydropyridine-4-oxyl)-coumarin
[0044]
[0045] The method and conditions are the same as in Example 1, except that 0.6 mmol (85.2 mg) of methyl iodide is changed to 0.6 mmol (100.8 mg) of acryloyl iodide, and other operations and methods are the same as in Example 1 to obtain 4-ene Propyl-7-(1-methyl-1,2,3,6-tetrahydropyridine-4-oxyl)-coumarin 26.5 mg, yield 89.2%, purity 99.7%.
[0046] 1HNMR (CDCl3, 500MHz)
[0047] δ2.42-2.43(d, 3H, J=1.0Hz), 2.43-2.47(s, 2H), 2.80-2.86(t, 2H, J=5.5Hz), 3.15-3.12(s, 2H), 3.21- 3.25(d, 2H, J=6.5Hz), 5.15-5.153(t, 1H, J=3.5Hz), 5.23-5.32(m, 2H, J=4.8Hz), 5.92-6.02(m, 1H, J= 4.2Hz), 6.18-6.22(s, 1H), 6.98-7.00(d, 1H, J=2.5Hz), 7.01-7.05(m, 1H, J=3.7Hz), 7.54-7.57(d, 1H, J =9.0Hz)
[0048] 13CNMR (150MHz, CDCl3)
[0049] 26.57(CH3), 49.03(CH2), 50.39(CH2), 60.28(CH2), 76.75(C), 77.00(C), 77.26(C), 105.70(C), 106.72(C), 112.46(C), 114.87(C), 115.01(CH), 125.59(CH), 133....
Embodiment 3
[0050] Example 3 Preparation of 4-benzyl-7-(1-methyl-1,2,3,6-tetrahydropyridine-4-oxyl group)-coumarin
[0051]
[0052] The same method and conditions as in Example 1, the difference is that 0.6mmol (85.2mg) methyl iodide is changed to 0.6mol (130.8mg) benzyl iodide, and other operations and methods are the same as in Example 1 to obtain 4-benzyl iodide 7-(1-methyl-1,2,3,6-tetrahydropyridine-4-oxyl)-coumarin 31.2 mg, yield 90%, purity 99.3%.
[0053] 1HNMR (CDCl3, 500MHz)
[0054] δ2.38-2.43(m, 3H, J=1.0Hz), 2.43-2.46(d, 2H, J=2.5Hz), 2.78-2.88(s, 2H), 3.16-3.22(s, 2H), 3.72- 3.80(d, 2H, J=4.0Hz), 5.14-5.18(t, 1H, J=3.5Hz), 6.18-6.21(d, 1H, J=1.0Hz), 6.96-7.00(d, 1H, J= 1.0Hz), 7.01-7.05(m, 1H, J=4.0Hz), 7.30-7.34(t, 1H, J=2.5Hz), 7.35-7.398(t, 2H, J=7.0Hz), 7.41-7.45( d, 2H, J=7.0Hz), 7.55-7.57 (d, 1H, J=8.5Hz)
[0055] 13CNMR (150MHz, CDCl3),
[0056] δc161.04(CH), 159.37(C), 154.88(C), 152.43(C), 150.46(C), 157.92(C), 129.14(CH), 128.37(CH), 127.29(CH), 125.75(CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com