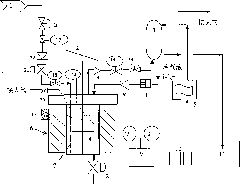
Preparation method of carboxylated lignin
A technology of lignin and carboxylation, which is applied in the field of low-carbon preparation of new materials and the recycling and reuse of renewable resources, can solve the problems of restricting, reducing the market demand of lignin-based low-efficiency water reducers, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The amount of catalyst in the reaction is 5.0wt.% of the total amount of lignin, containing Fe 2+ (Iron acetylacetonate), Cu 2+ (Schiff base copper), and Nd 3+ (Neodymium acetate) salt, molar ratio Fe 2+ / Cu 2+ / Nd 3+ =6 / 4 / 0.5, dissolved in 30wt% lignin aqueous solution. The reaction pressure is 0.02MPa, the reaction temperature is 90°C, and the reaction time is 2 hours. The experimental results show that the carboxyl (-COOH) content of the product has increased by 8×10 -3 mol / g, compared with the original lignin, the molecular weight distribution curve measured by GPC moved to the high molecular weight end, from 20,000 to 40,000 of the original lignin, and the molecular weight distribution narrowed, and the peak half-maximum width (HWFM) from 30,000 dropped to 21,000.
Embodiment 2
[0026] The amount of catalyst in the reaction is 3.0wt.% of the total lignin, containing Sn 4+ (tin octoate), Ti 4+ (titanium naphthenate), and Po 3+ (Po(iPrCp)) salt with a molar ratio of Sn 4+ / Ti 4+ / Po 3+ =4 / 1 / 0.5, dissolved in 20wt% lignin aqueous solution. The reaction pressure is 0.3 MPa, the reaction temperature is 110° C., and the reaction time is 30 minutes. The experimental results show that the carboxyl (-COOH) content of the product has increased by 6×10 -3 mol / g, compared with the original lignin, the molecular weight distribution curve measured by GPC moved to the high molecular weight end, from 20,000 to 30,000 of the original lignin, and the molecular weight distribution narrowed, and the peak half-maximum width (HWFM) from 30,000 dropped to 25,000.
Embodiment 3
[0028] The amount of catalyst in the reaction is 3.0wt.% of the total amount of lignin, containing V 4+ (vanadium acetate), Zr 4+ (zirconium naphthenate), and Er 3+ (Erbium acetylacetonate) salt, the molar ratio is V 4+ / Zr 4+ / Er 3+ =5 / 1 / 2, dissolved in 10wt% lignin aqueous solution. The reaction pressure is 0.5 MPa, the reaction temperature is 140° C., and the reaction time is 4 hours. The experimental results show that the carboxyl (-COOH) content of the product has increased by 20×10 -3 mol / g, compared with the original lignin, the molecular weight distribution curve measured by GPC moves to the end of high molecular weight and low molecular weight, from 20,000 to 10,000 of the original lignin, and the molecular weight distribution becomes narrower, and the peak half-maximum width (HWFM ) from 30,000 to 10,000.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com