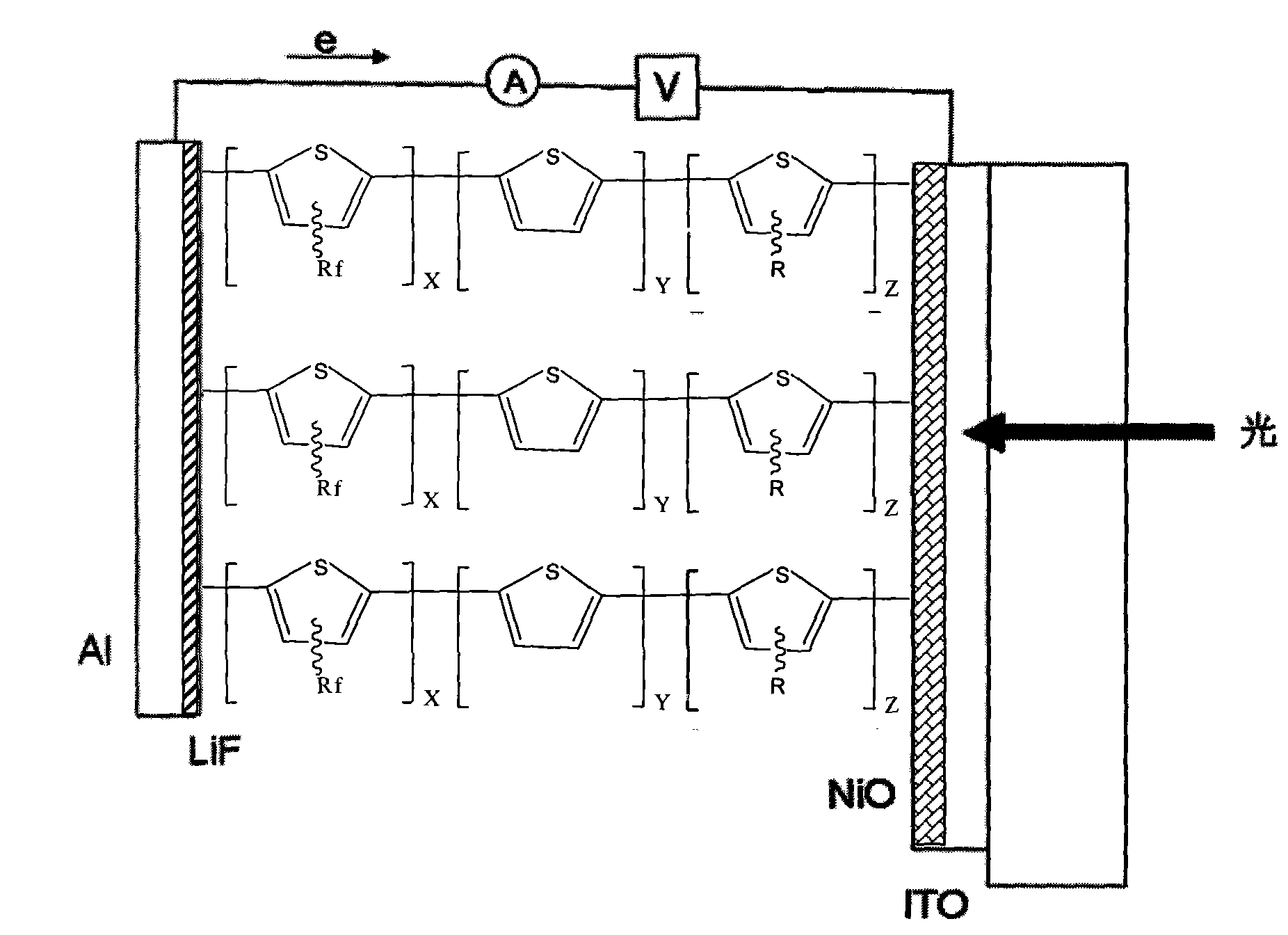
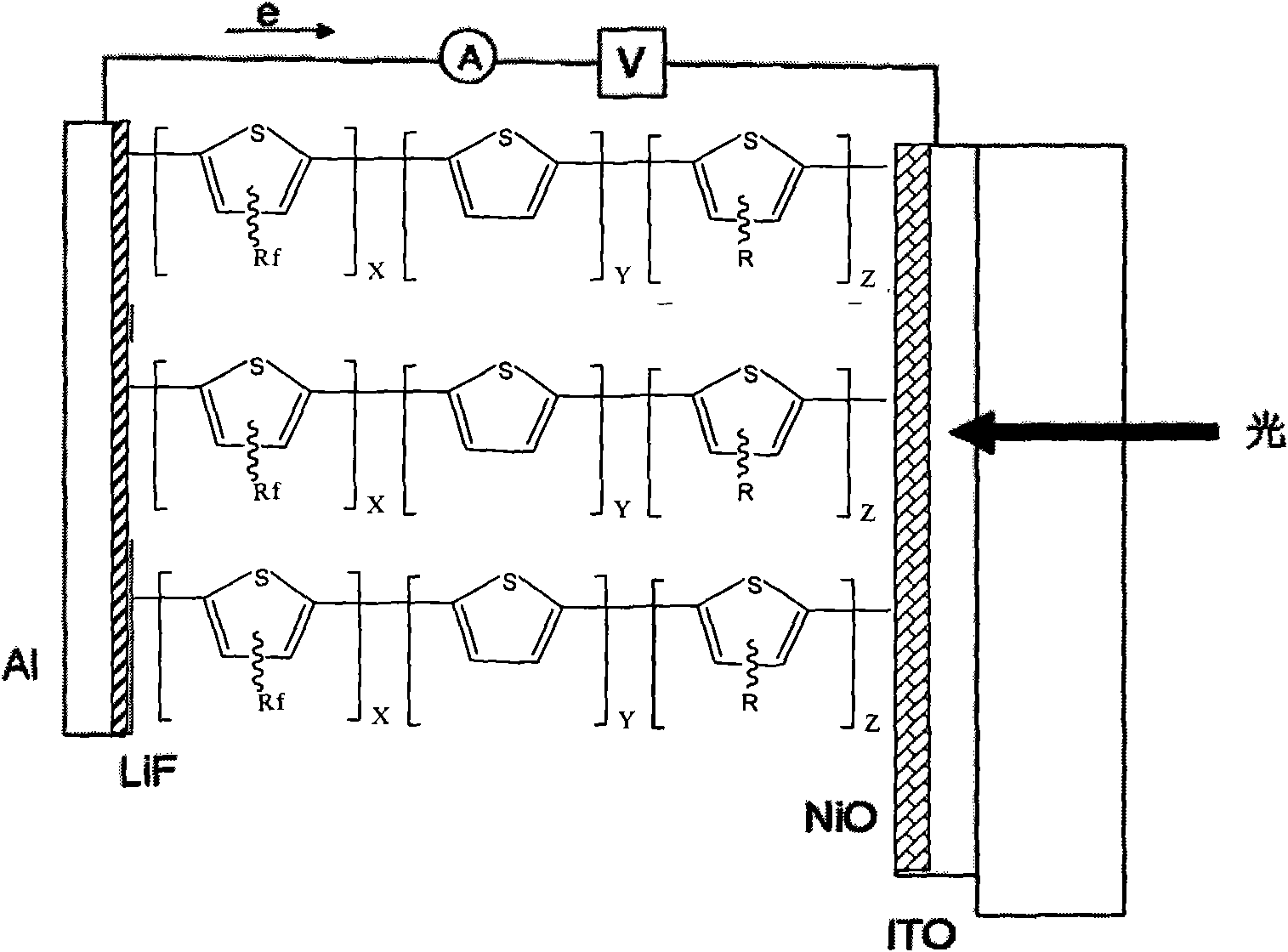
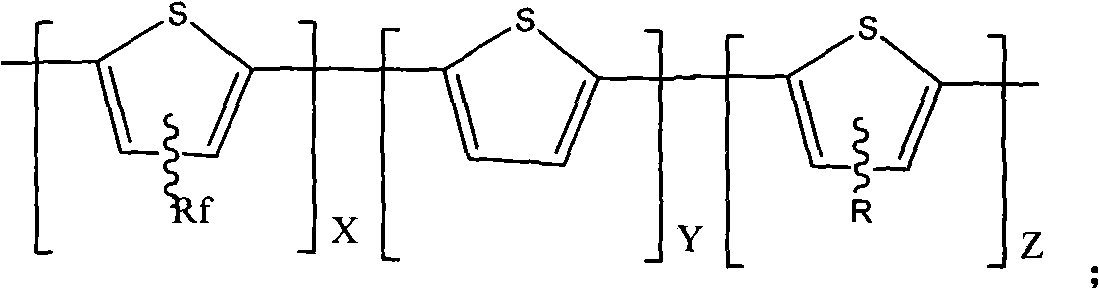
Fluorine-contained polythiophene photoelectric material with high optical conversion rate and preparation method thereof
A photoelectric material, polythiophene technology, applied in luminescent materials, chemical instruments and methods, photovoltaic power generation, etc., can solve problems such as low photoelectric conversion efficiency of organic thin film solar cells, low potential for improving photoelectric conversion efficiency, and silicon material supply crisis. Achieve the effect of improving electron acceptability, less defects, and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] A kind of preparation method of the polythiophene photoelectric material of high light conversion rate of the present invention comprises the following steps:
[0020] Step 1, synthesizing fluorine-containing alkyl chain thiophene monomer: using coupling reaction or substitution reaction to realize fluoroalkylation of thiophene;
[0021] Step 2. Synthesis of fluorine-containing alkyl chain polythiophene macromolecules: using active catalytic transfer Grignard reaction to polymerize the fluorine-containing alkyl chain thiophene monomers synthesized in step 1 into fluorine-containing alkyl chain polythiophene macromolecules;
[0022] Step 3, the process of synthesizing the tri-block polythiophene macromolecules: the step-by-step catalyst transfer polymerization method is used to polymerize the fluorine-containing alkyl chain polythiophene macromolecules synthesized in step 2 into tri-block polythiophene macromolecules.
[0023] Among them, step 1 can be realized through t...
Embodiment 1
[0029] Step 1, synthesis of fluorine-containing alkyl chain thiophene monomer:
[0030] In the presence of copper powder, 3-bromothiophene (1) and perfluorohexyl iodide were heated and stirred in dimethyl sulfoxide (DMSO), and the coupling reaction was carried out to generate 3-perfluorohexylthiophene (2 ), the specific coupling reaction conditions can refer to the 4718-4719 pages of the 16th issue of Chemistry of Materials in 2004; after separation and purification, the generated product can be further brominated with NBS (N-Bromosuccinimide), and 2,5- Dibromo, 3-perfluorohexylthiophene (3) is separated and purified, and further brominated with NBS (N-Bromosuccinimide) to obtain 2,5-dibromo, 3-perfluorohexylthiophene (3) ). The synthetic route is shown below.
[0031]
[0032] Step 2, synthesis of fluorine-containing alkyl chain polythiophene macromolecule:
[0033] Utilize 2,5-dibromo, 3-perfluorohexylthiophene (3) synthesized in step 1 as a raw material, first react w...
Embodiment 2
[0043] Step 1, synthesis of fluorine-containing alkyl chain thiophene monomer:
[0044] 3-Bromothiophene 1 first generates 3-thiophene lithium (4) with butyllithium; 3-thiophene lithium (4) undergoes a substitution reaction with perfluorohexanesulfonyl fluoride or acyl fluoride at low temperature to generate 3-perfluorohexyl Alkylsulfonylthiophene (5), or 3-acylthiophene (6), the specific substitution reaction conditions can refer to the document Organic Electronics 2010, No. 11, pages 801-813; the generated 3-perfluorohexanesulfonyl After thiophene (5) or 3-acylthiophene (6) is separated and purified, it is further brominated with NBS to obtain 2,5-dibromo, 3-perfluorohexanesulfonylthiophene (7) or 2,5-dibromo , 3-acylthiophene (8). The synthetic route is shown below.
[0045]
[0046] Step 2, the process of synthesizing fluorine-containing alkyl chain polythiophene macromolecule:
[0047] Utilize the brominated thiophene monomer (7) or (8) containing the fluorine-conta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com