HPLC (High Performance Liquid Chromatography) method for measuring content of D-4-methylsulfonylphenyl serine ethyl ester
A technology of thiamphenylphenylserine ethyl ester and content, which is applied in the field of pharmaceutical and chemical quality inspection, and achieves the effects of good specificity, good reproducibility, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
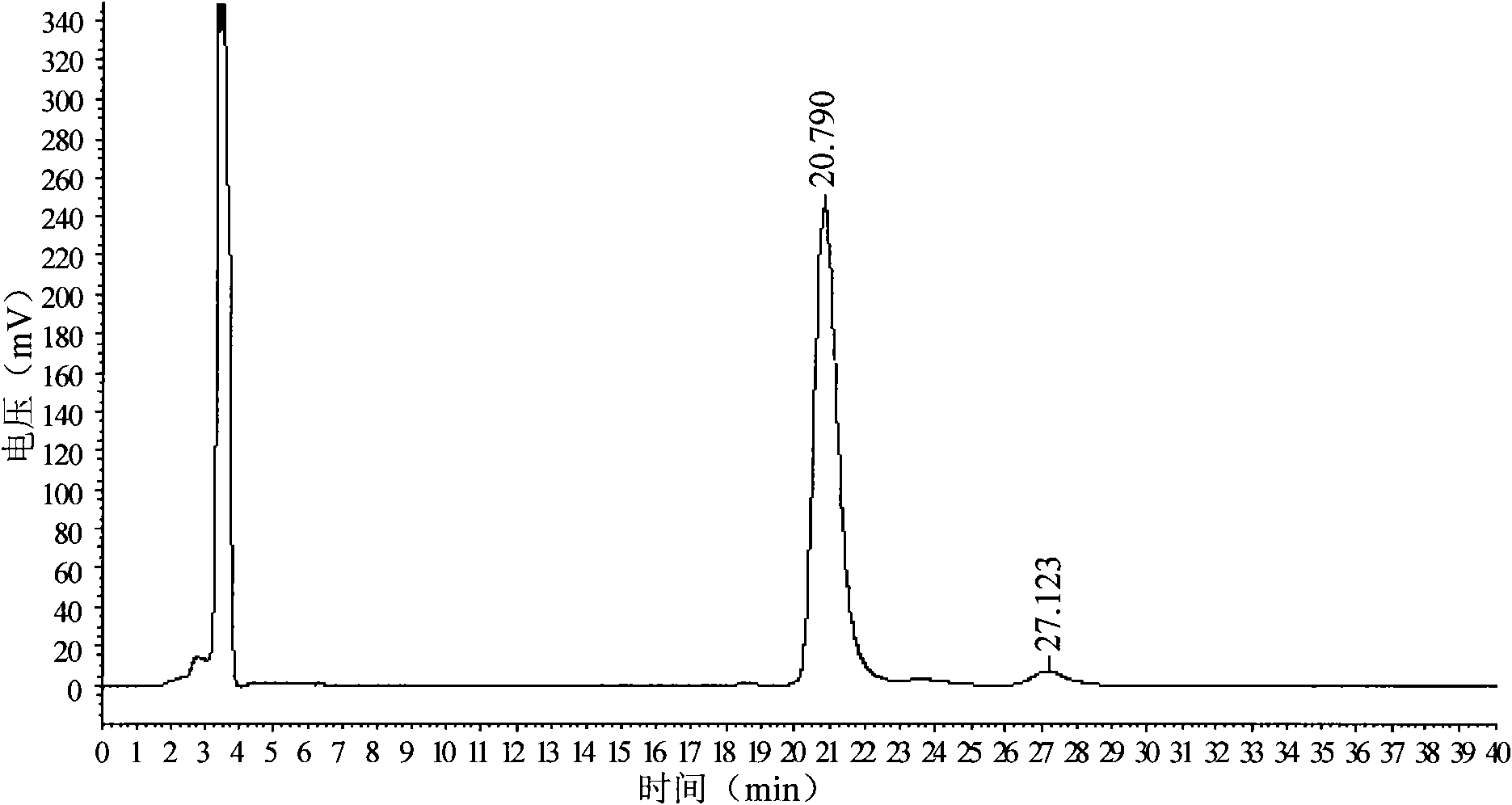
[0027] Chiral column with cellulose-tris(4-methylbenzoate) stationary phase coated on silica gel surface, column length 150mm×inner diameter 4.6mm×filler particle size 5μm, mobile phase is n-hexane: acetonitrile: ethylenediamine =40:60:0.1, the flow rate is 0.4ml / min, the detection wavelength is 210nm, the column temperature is 32°C, and the injection volume is 5μL. Accurately weigh 25 mg of D-p-methylsulfonyl phenylserine ethyl ester sample (batch: 090501) into a 25 ml volumetric flask, add an appropriate amount of n-propanol: methanol (1:1, v / v) solvent to dissolve it by ultrasound, and then Add the above-mentioned mixed solvent of n-propanol and methanol to dilute to the mark, and filter with 0.22μm filter membrane before sample injection for chromatographic analysis.
[0028] Take the above-mentioned solution to be tested, inject it into the chromatograph, measure twice in parallel, record the detection time for 40 minutes, obtain the chromatogram, calculate the content of D-...
Embodiment 2
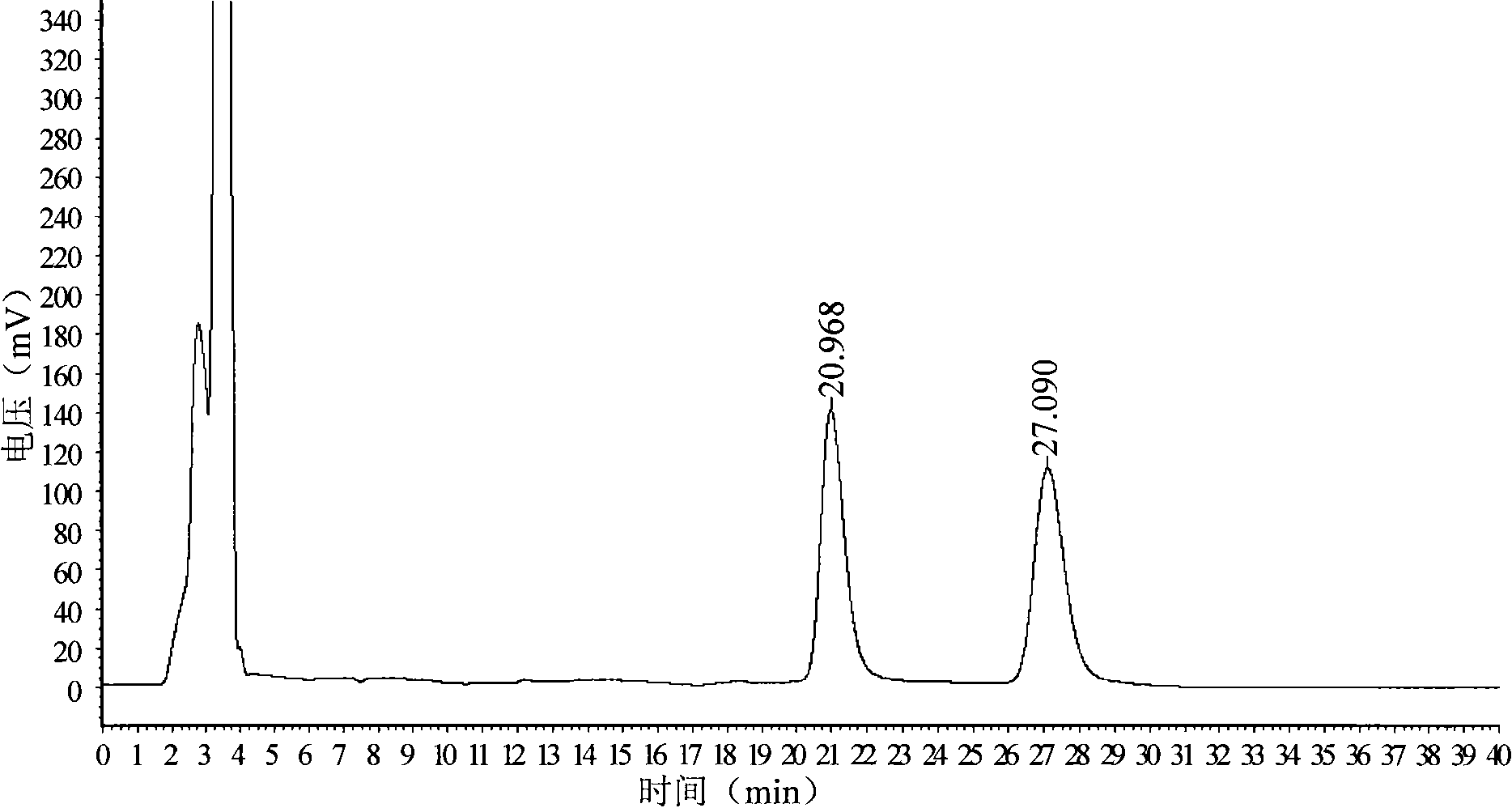
[0030] Chiral column with cellulose-tris(4-methylbenzoate) stationary phase coated on silica gel surface, column length 150mm×inner diameter 4.6mm×filler particle size 10μm, mobile phase is n-hexane: methanol: ethylenediamine =60:40:0.8, the flow rate is 1.5ml / min, the detection wavelength is 235nm, the column temperature is 39°C, and the injection volume is 20μL. Accurately weigh 25 mg of D-p-methylsulfonyl phenylserine ethyl ester sample (batch: 090502) into a 25ml volumetric flask, add an appropriate amount of n-propanol: methanol (1:1, v / v) solvent to dissolve it by ultrasound, and then Add the above-mentioned mixed solvent of n-propanol and methanol to dilute to the mark, filter with 0.45μm filter membrane before sample injection, for chromatographic analysis.
[0031] Take the above-mentioned solution to be tested, inject it into the chromatograph, measure twice in parallel, record the detection time for 40 minutes, obtain the chromatogram, calculate the content of D-p-meth...
Embodiment 3
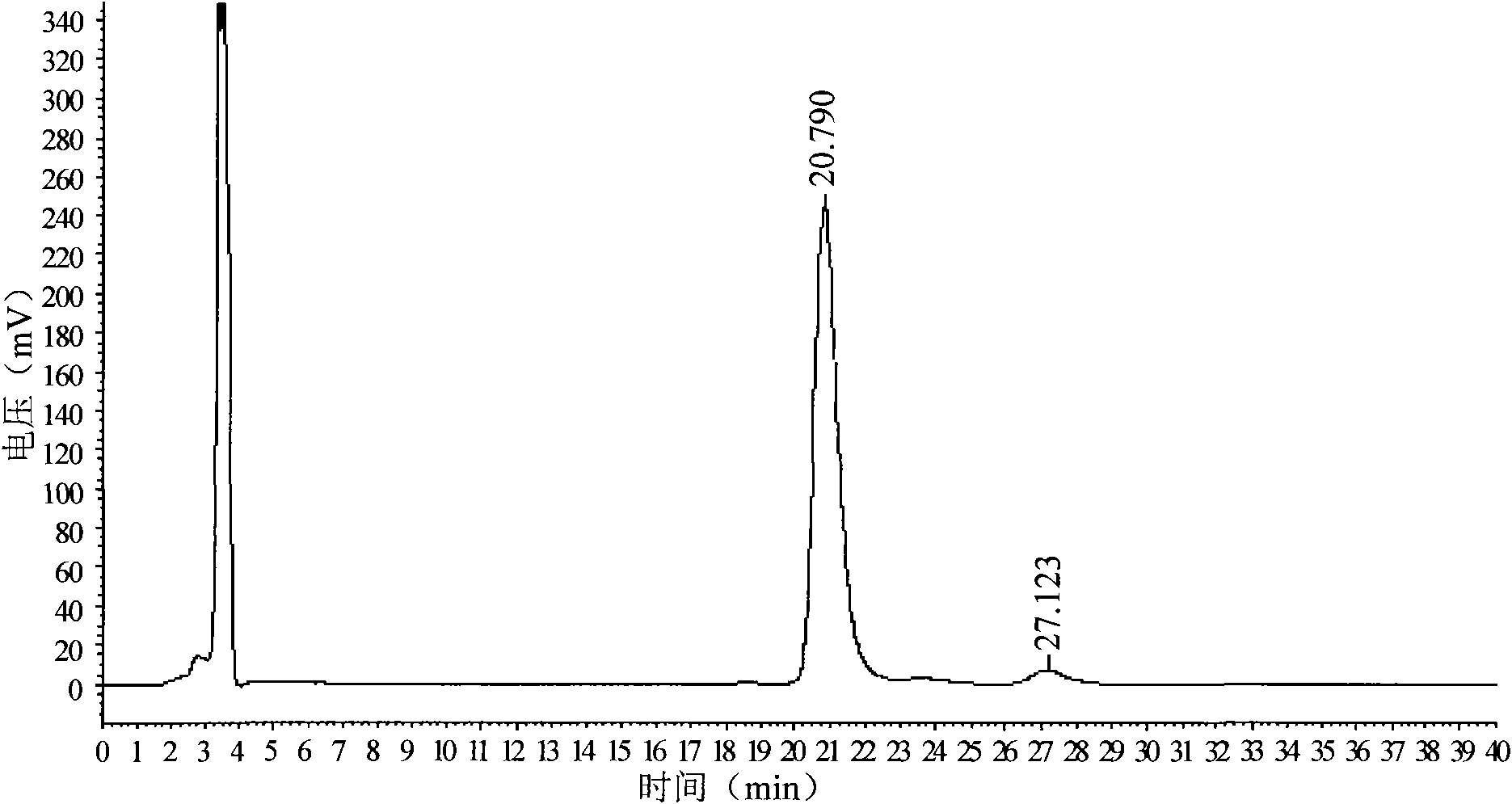
[0033] A chiral column with cellulose-tris(4-methylbenzoate) stationary phase coated on silica gel surface, column length 250mm×inner diameter 4.6mm×filler particle size 5μm, mobile phase is n-hexane: absolute ethanol: two Ethylamine=60:40:0.5, the flow rate is 1.2ml / min, the detection wavelength is 220nm, the column temperature is 35°C, and the injection volume is 20μL. Accurately weigh 25mg of D-p-methylsulfonylphenylserine ethyl ester sample (batch: 090503) into a 25ml volumetric flask, add an appropriate amount of n-propanol: methanol (1:1, v / v) solvent to dissolve it by ultrasound, and then Add the above-mentioned mixed solvent of n-propanol and methanol to dilute to the mark, filter with 0.45μm filter membrane before sample injection, for chromatographic analysis.
[0034] Take the above-mentioned solution to be tested, inject it into the chromatograph, measure twice in parallel, record the detection time for 40 minutes, obtain the chromatogram, calculate the content of D-p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com