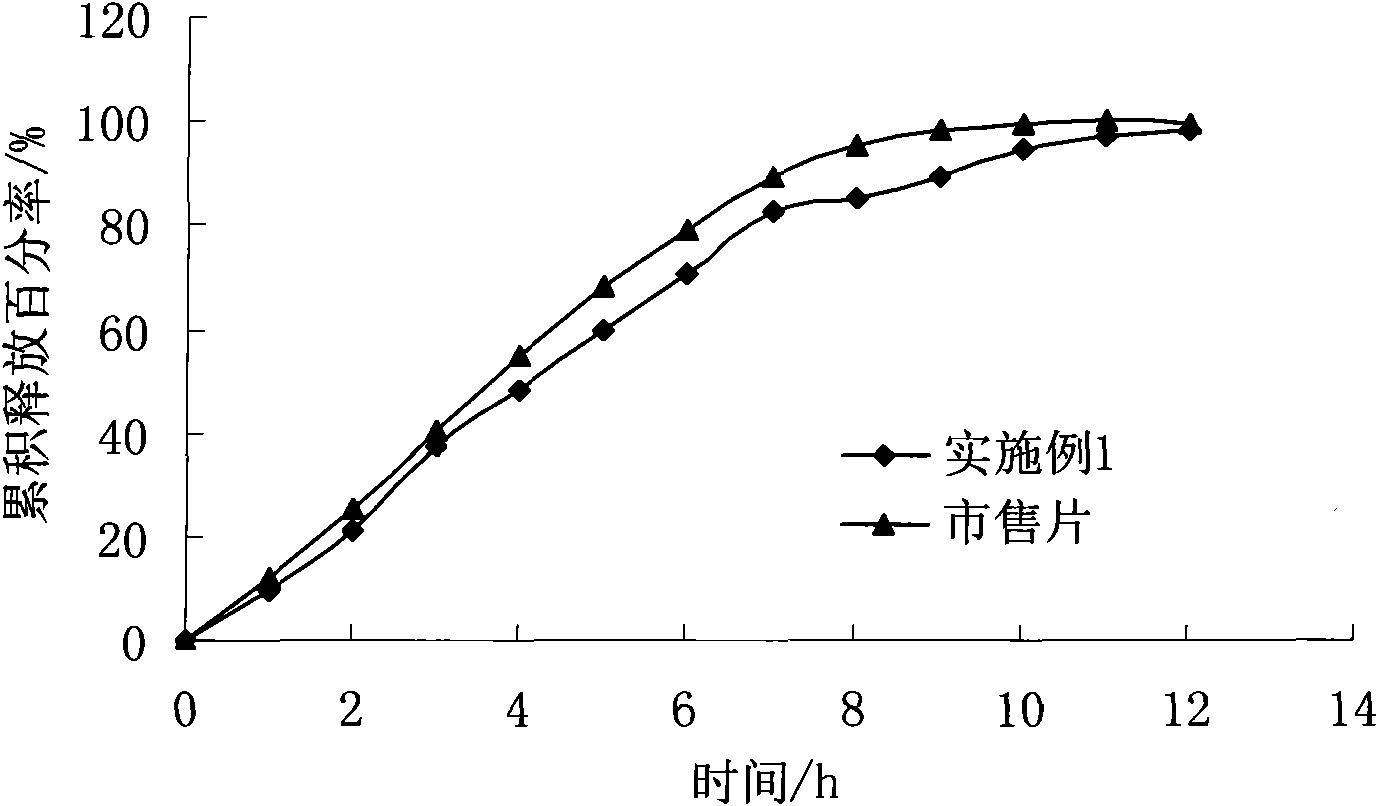
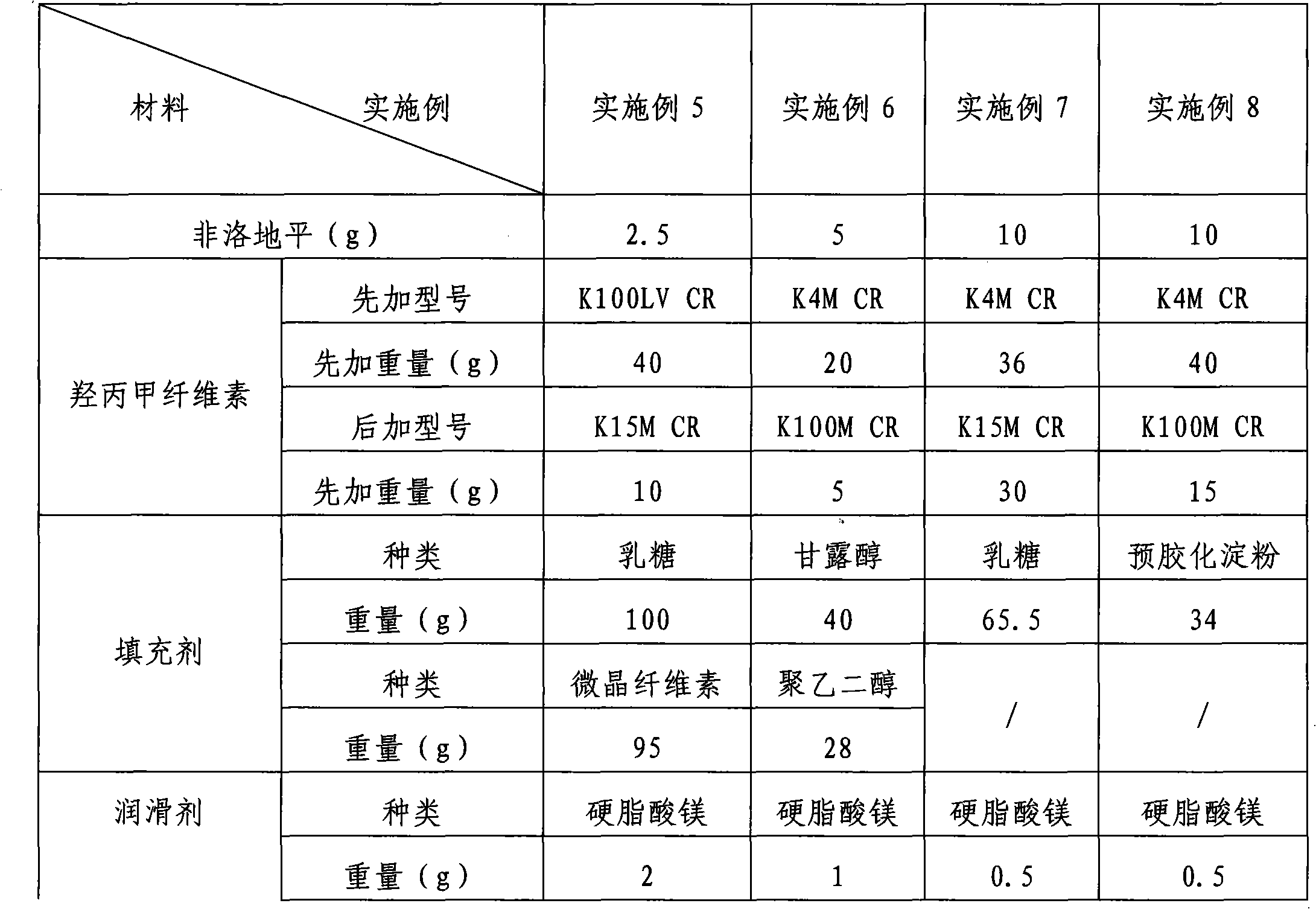
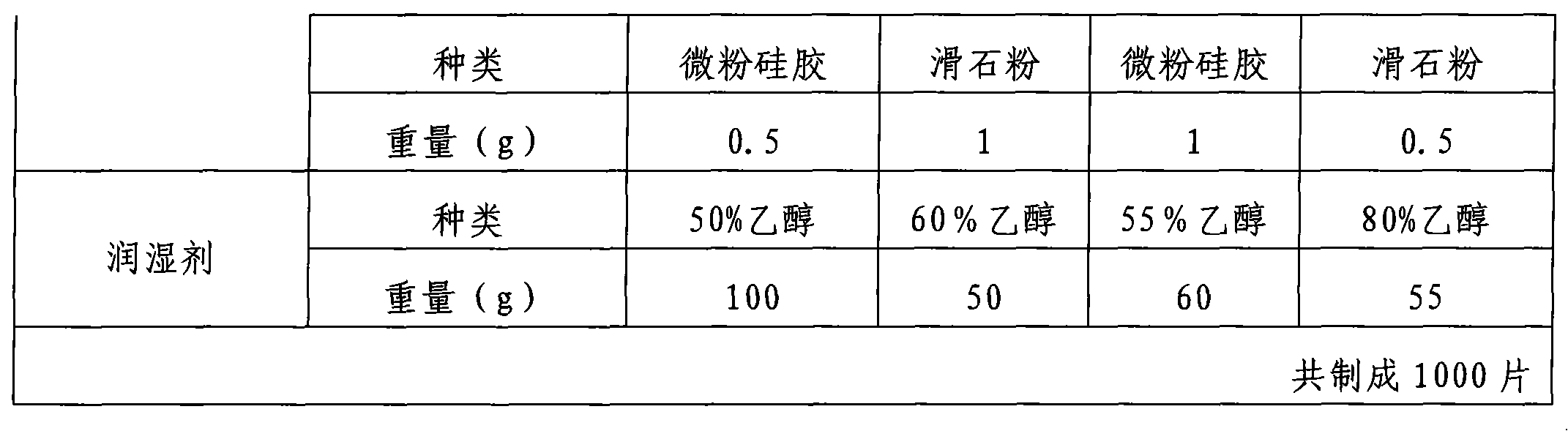
Preparation method of felodipine sustained release tablets
A felodipine and gentle technology, applied in the field of preparation of felodipine sustained-release tablets, can solve the problems of cumbersome process, difficult industrial production, high production cost, etc., achieve high product yield, easy preparation method, and reduce production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of felodipine sustained-release tablets
[0029] prescription:
[0030] Felodipine 5g
[0031] Hypromellose K100LV CR 50g
[0032] Hypromellose K4M CR 28g
[0033] Lactose 115g
[0035] 65% ethanol appropriate amount
[0036] 1000 pieces in total
[0037] Preparation Process:
[0038] 1. Preparation of tablet core: Weigh the prescription amount of felodipine, hypromellose K100LV CR, lactose, mix well, add about 80g of 65% ethanol solution to make soft material, sieve to make wet granules, 50-60 It is dried at °C to a water content of 2 to 3%, granulated, and a prescription amount of hypromellose K4M CR, magnesium stearate is added, and tableted to obtain a tablet core. The content of felodipine is 2.5%.
[0039] 2. Coating: Use Opadry for pan coating, increase the weight by 2 to 3%, and make a sustained-release tablet containing 5 mg of felodipine.
Embodiment 2
[0041] Felodipine 5g
[0042] Hypromellose K100LV CR 29.4g
[0043] Hypromellose K4MCR 10.3g
[0044] Hypromellose K15M CR 11.8g
[0045] Mannitol 59.7g
[0046] Polyethylene glycol 6000 29.4g
[0047] Magnesium stearate 0.7g
[0048] Talc 0.7g
[0049] 65% ethanol appropriate amount
[0050] 1000 pieces in total
[0051] Preparation Process:
[0052] 1. Preparation of the tablet core: Weigh the prescription amount of felodipine, hypromellose K100LV CR, mannitol, and polyethylene glycol 6000 and mix them evenly, add about 65g of a 65% ethanol solution to make a soft material, sieving, Make wet granules, dry at 50~60℃ to 2~3% LOD, sizing, add prescription amount of hypromellose K4M CR, hypromellose K15M CR, magnesium stearate and talc, and press , Control the film hardness to 4~7kg to obtain the tablet core. The main drug content is 3.3%.
[0053] 2. Coating: Use Opadry and pan coating until the tablet core increases to 2 to 3% to make a sustained-release tablet containing ...
Embodiment 3
[0055] Felodipine 2.5g
[0056] Hypromellose K4M CR 15g
[0057] Hypromellose K15M CR 5g
[0058] Lactose 16g
[0059] Pregelatinized starch 33g
[0060] Microcrystalline cellulose 27.5g
[0061] Magnesium stearate 0.5g
[0062] Micro powder silica gel 0.5g
[0063] 70% ethanol appropriate amount
[0064] 1000 pieces in total
[0065] Preparation Process:
[0066] 1. Preparation of tablet core:
[0067] Weigh the prescription amount of felodipine, hypromellose K4M CR, lactose, pregelatinized starch and microcrystalline cellulose, mix them evenly, add about 50g of 70% ethanol solution to make soft material, and sieve to make wet granules , Dried at 50~60℃ until the LOD is 2~3%, sieved and granulated, added the prescribed amount of hypromellose K15M CR, magnesium stearate and finely powdered silica gel, compressed into tablets, controlled the tablet hardness to 3~5kg, Obtain the core. The main drug content is 2.5%.
[0068] 2. Coating: Use Opadry and pan coating until the tablet ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com