Acid-sensitive polymeric micelle pharmaceutical composition and preparation method thereof
A technology of polymers and synthetic methods, applied in the direction of drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve the problems of loss, toxic side effects, short half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
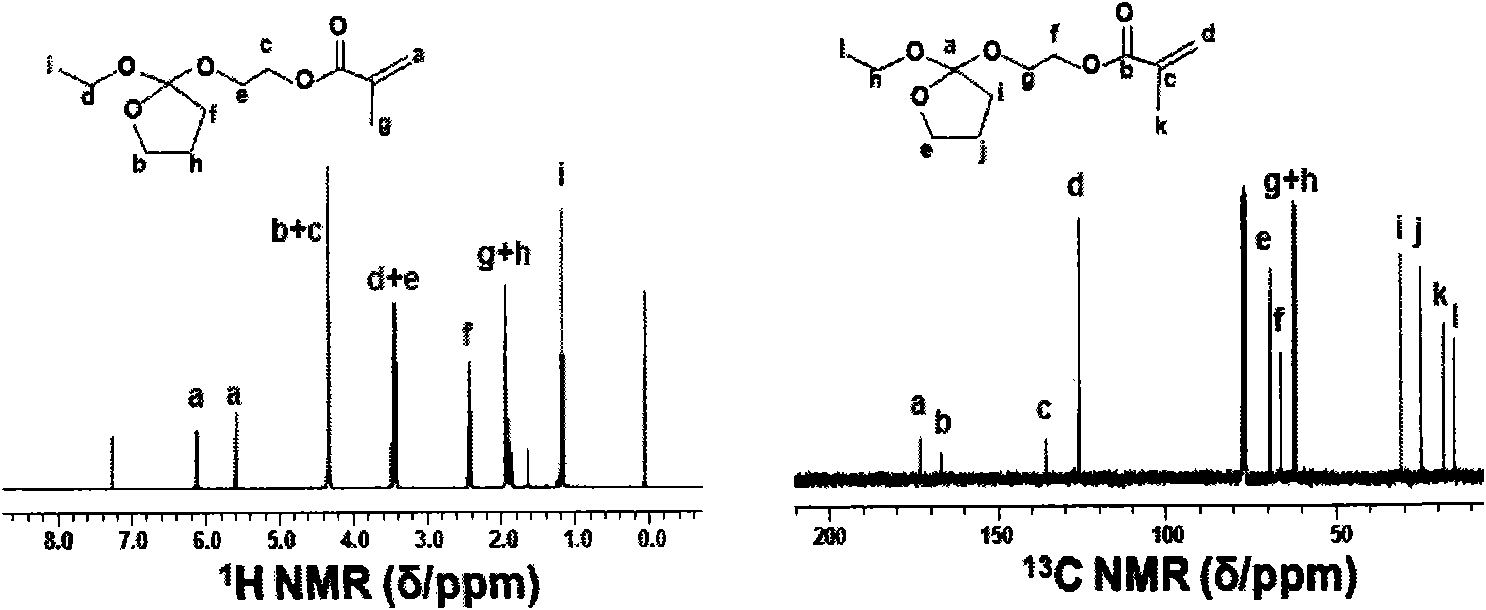
[0044] The synthetic method of 2-ethoxytetrahydrofuran-2-oxyethyl methacrylate monomer is realized through the following steps:
[0045] 1) Preparation of 2-(2'-hydroxyethoxy)-2-ethoxytetrahydrofuran
[0046] Under nitrogen atmosphere, 7.60g (47.44mmol) of 2,2-diethoxytetrahydrofuran, 11.78g (189.79mmol) of ethylene glycol and a small amount of p-toluenesulfonic acid were reacted at 130°C for 18 hours and then cooled to room temperature. The reaction mixture was dissolved in ethyl acetate, washed with saturated aqueous sodium carbonate and saturated brine, dried over magnesium sulfate, evaporated under reduced pressure to remove the solvent, and dried in vacuo to obtain 6.94 g of a colorless oily pure product with a yield of 83%. 1 H NMR (300MHz, CDCl 3 ): δ (ppm) 1.15-1.20 (t, 3H, CH 3 ), 1.89-1.94 (m, 2H, CH 2 ), 2.42-2.47 (t, 2H, CH 2 ), 3.42-3.61 (m, 4H, OCH 2 ), 3.69-3.82 (m, 2H, CH 2 OH), 4.19-4.23(t, 2H, OCH 2 ). 13 C NMR (CDCl 3 , δppm): 15.22, 25.15, 30.97, 3...
Embodiment 2
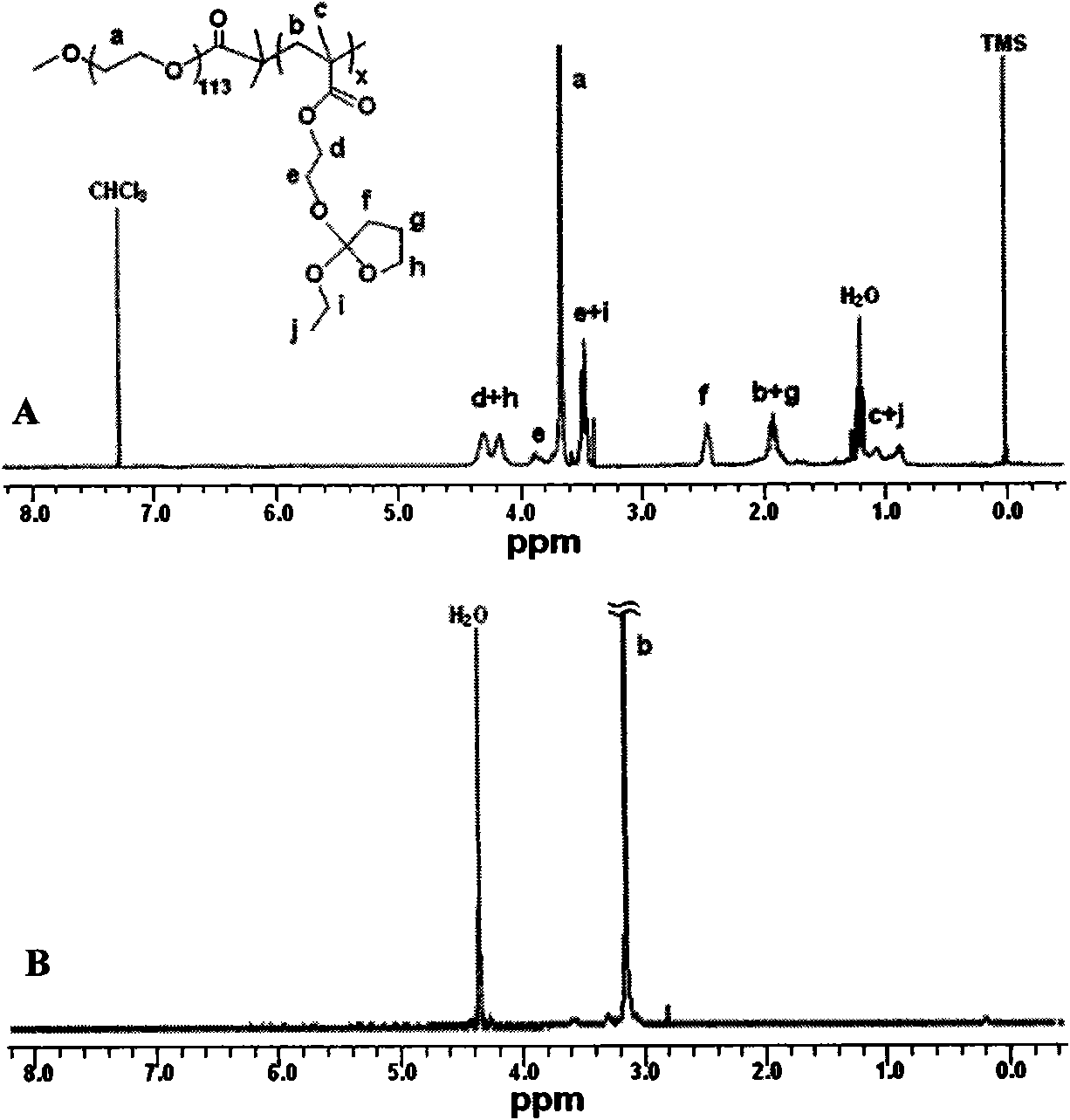
[0050] 1.50g (0.29mmol) polyethylene glycol macroinitiator, 2.14g (8.76mmol) 2-ethoxytetrahydrofuran-2-oxyethyl methacrylate, 28.86mg (0.29mmol) cuprous chloride, 90.58mg (0.58mmol) of bipyridine was accurately weighed and put into a dry and clean glass polymerization tube, and finally 4ml of toluene was added. After the polymer was frozen three times, thawed, vacuumed, nitrogen-filled and deoxidized, the tube was sealed, and then the polymerization reaction tube was heated and polymerized in an oil bath at 80°C. After 8 hours of reaction, the reaction mixture was dissolved in tetrahydrofuran and passed through a short period of time. A basic alumina column to remove metal compounds from the catalyst. After most of the tetrahydrofuran was removed by rotary evaporation, the polymer was precipitated with a large amount of hexane, filtered, and vacuum-dried to constant weight to obtain the target copolymer P1. The properties of the copolymer are shown in Table 1.
Embodiment 3
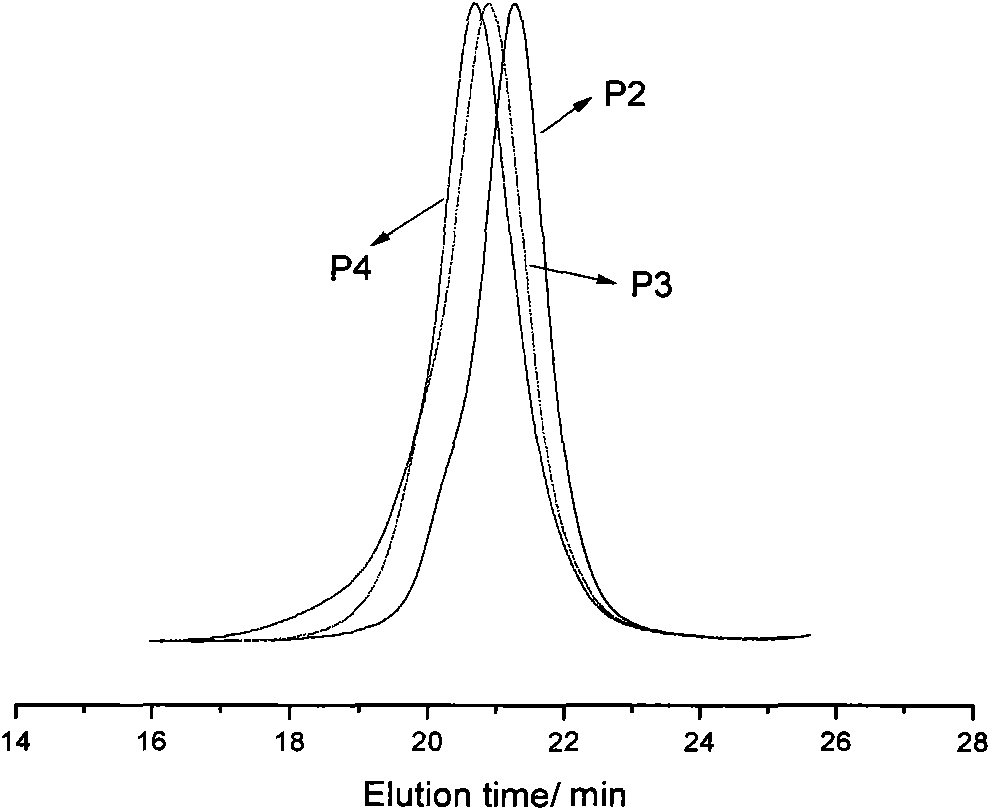
[0052] 1.50g (0.29mmol) polyethylene glycol macroinitiator, 3.57g (14.60mmol) 2-ethoxytetrahydrofuran-2-oxyethyl methacrylate, 28.86mg (0.29mmol) cuprous chloride, 90.58mg (0.58mmol) of bipyridine was accurately weighed and put into a dry and clean glass polymerization tube, and finally 5ml of toluene was added. After the polymer was frozen three times, thawed, vacuumed, nitrogen-filled and deoxidized, the tube was sealed, and then the polymerization reaction tube was heated and polymerized in an oil bath at 80°C. After 12 hours of reaction, the reaction mixture was dissolved in tetrahydrofuran and passed through a short period of time. A basic alumina column to remove metal compounds from the catalyst. After most of the tetrahydrofuran was removed by rotary evaporation, the polymer was precipitated with a large amount of hexane, filtered, and vacuum-dried to constant weight to obtain the target copolymer P2. The properties of the copolymer are shown in Table 1.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap