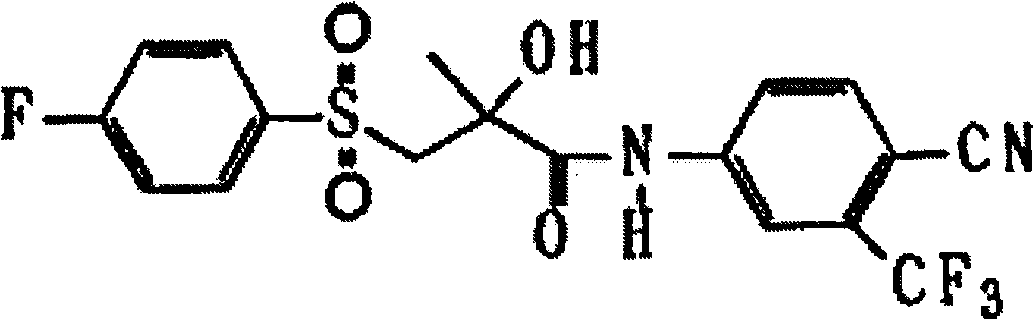
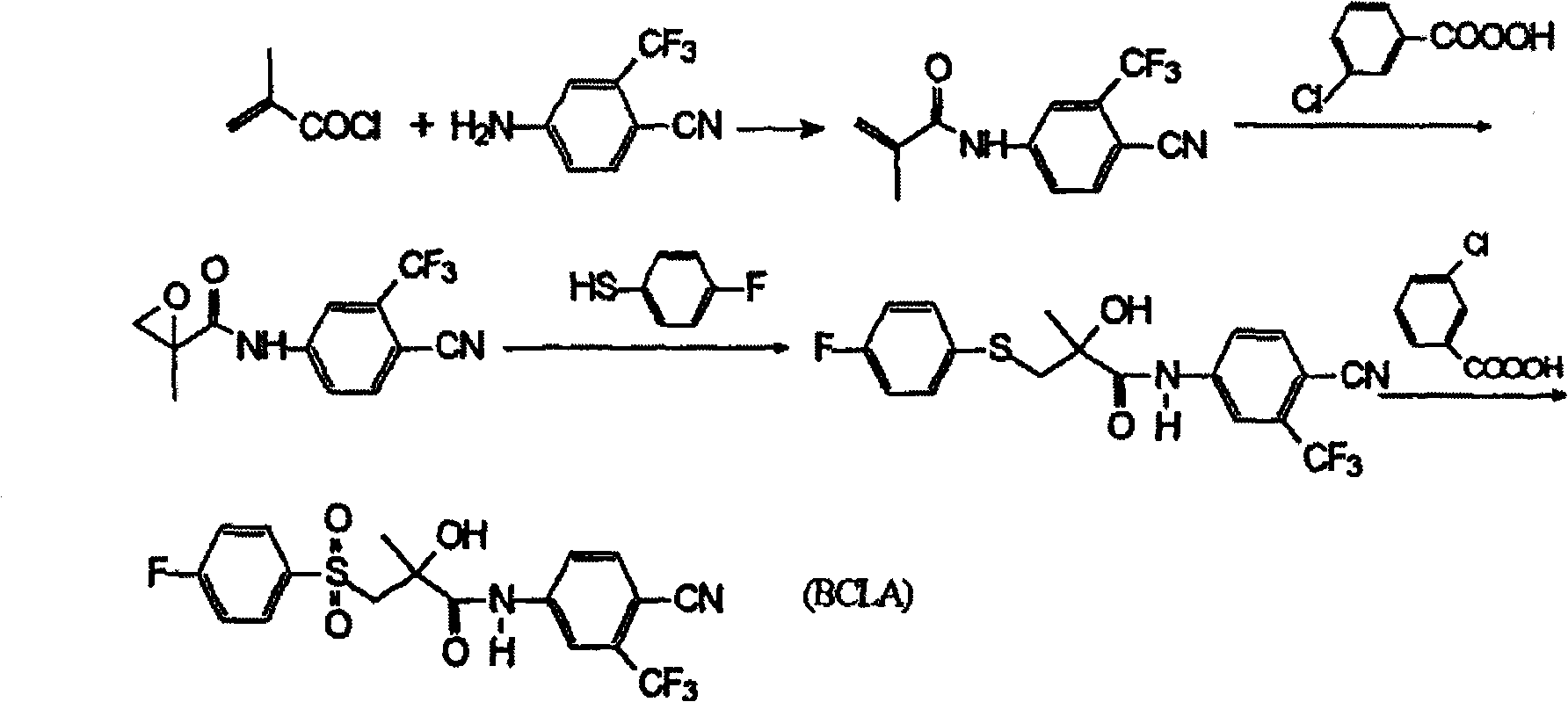
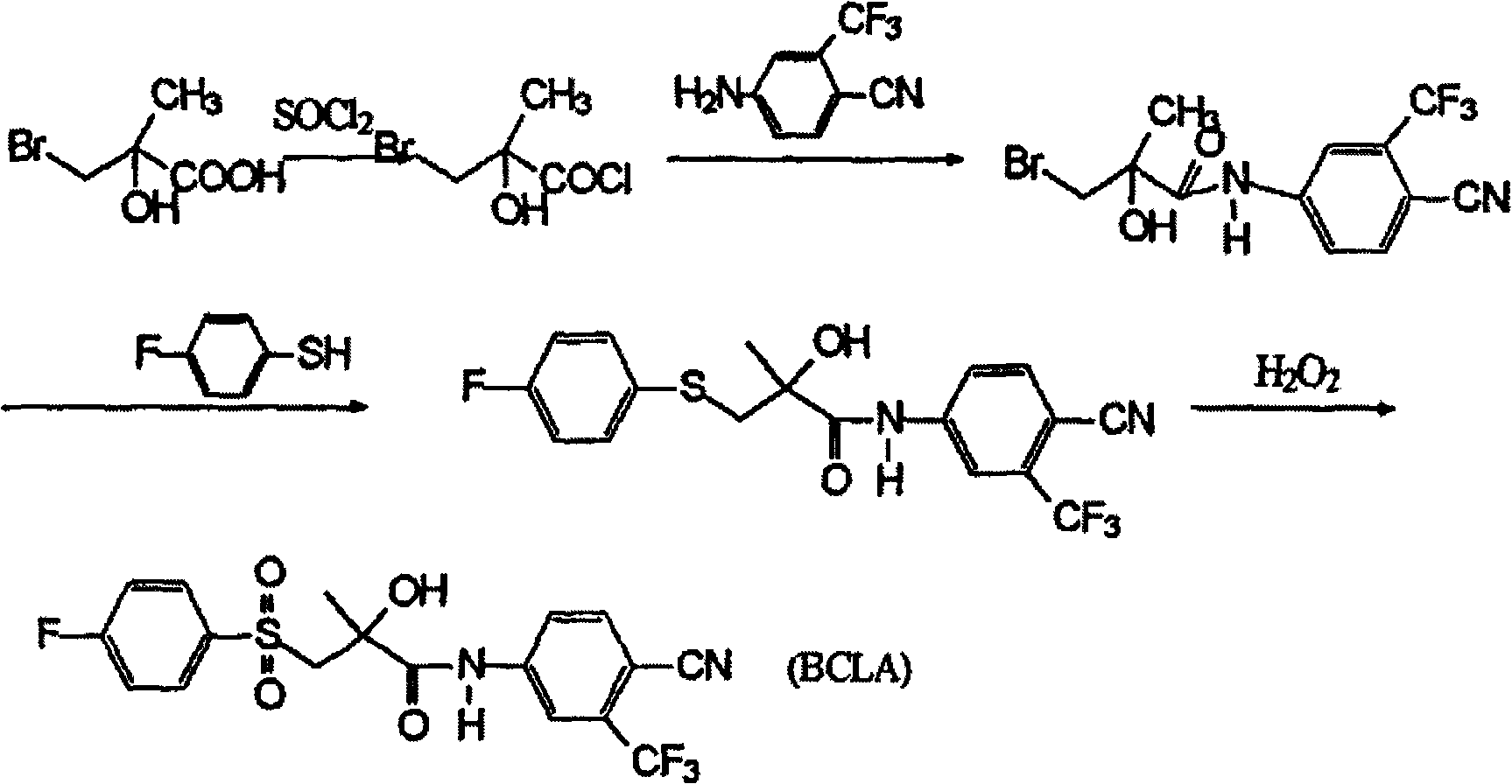
Preparation method of bicalutamide intermediate
A technology for bicalutamide and intermediates, which is applied in the field of preparation of anti-androgen drugs bicalutamide intermediates, can solve high problems, can spontaneously ignite in humid air, and is released when heated or in contact with moisture and acids. Heat and hydrogen, the quality of finished products is difficult to guarantee, and it is difficult to industrialize production, etc., to achieve the effect of low cost, good product quality and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation of embodiment 1, 4'-cyano-3-[(4-fluorophenyl) sulfur]-2-hydroxyl-2-methyl-3'-(trifluoromethyl)propionanilide
[0022] In the reaction vessel, add 20ml of dimethylformamide, 1,2-epoxy-2-methyl-N-[4'-cyano-3'-(trifluoromethyl)phenyl]propionamide 5g, four 0.10g of butyl ammonium bromide, 2g of potassium carbonate, stirring and dissolving, adding 2.8g of p-fluorothiophenol, slowly heating to 50-60°C for 16 hours, cooling to room temperature, adding 100ml of water, extracting with diethyl ether twice, anhydrous Na 2 SO 4 dry. The solvent was recovered to obtain 7.7 g of solid. Recrystallized from petroleum ether to obtain 6.7g, m.p 115-117°C, yield 91%.
Embodiment 2
[0023] Embodiment 2, the preparation of 4'-cyano-3-[(4-fluorophenyl)sulfur]-2-hydroxyl-2-methyl-3'-(trifluoromethyl)propionanilide
[0024] Add 20ml of dimethylformamide, 1,2-epoxy-2-methyl-N-[4'-cyano-3'-(trifluoromethyl)phenyl]propionamide 5g, carbonic acid Potassium 2.5g, stirred and dissolved, added 2.8g of p-fluorothiophenol, slowly heated to 50-60°C and reacted for 16 hours, and the remaining treatment methods were the same as in Example 1 to obtain 6.8g of solid, m.p115-116°C, yield 92.2 %.
Embodiment 3
[0025] Example 3, (R)-(-)4'-cyano-3-[(4-fluorophenyl)sulfanyl]-2-carbonyl-2-methyl-3'-(trifluoromethyl) Preparation of Propionanilide
[0026] Add 40ml of dimethylformamide to the reactor, (R)-(-)3-bromo-2-hydroxyl-2-methyl-N-[4-cyano-3-(trifluoromethylphenyl) ] Propionamide 12.7g, salt of wormwood 3g, stir and dissolve and add p-fluorothiophenol 4.9g, stir slowly and heat to 50~60 ℃ and react for 16 hours, all the other treatment methods are the same as embodiment 1, get solid 13.2g, m.p 93~ 96°C, yield 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com