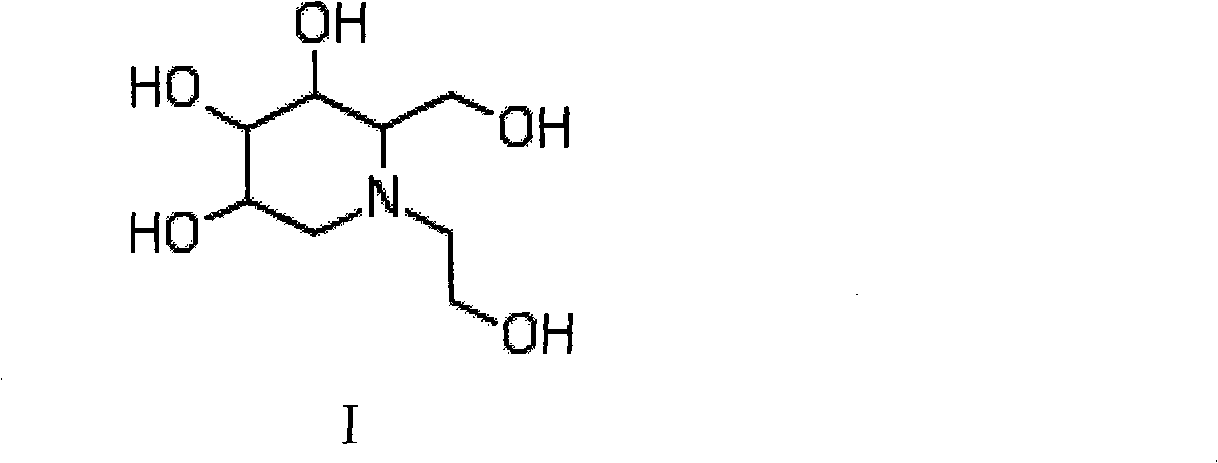
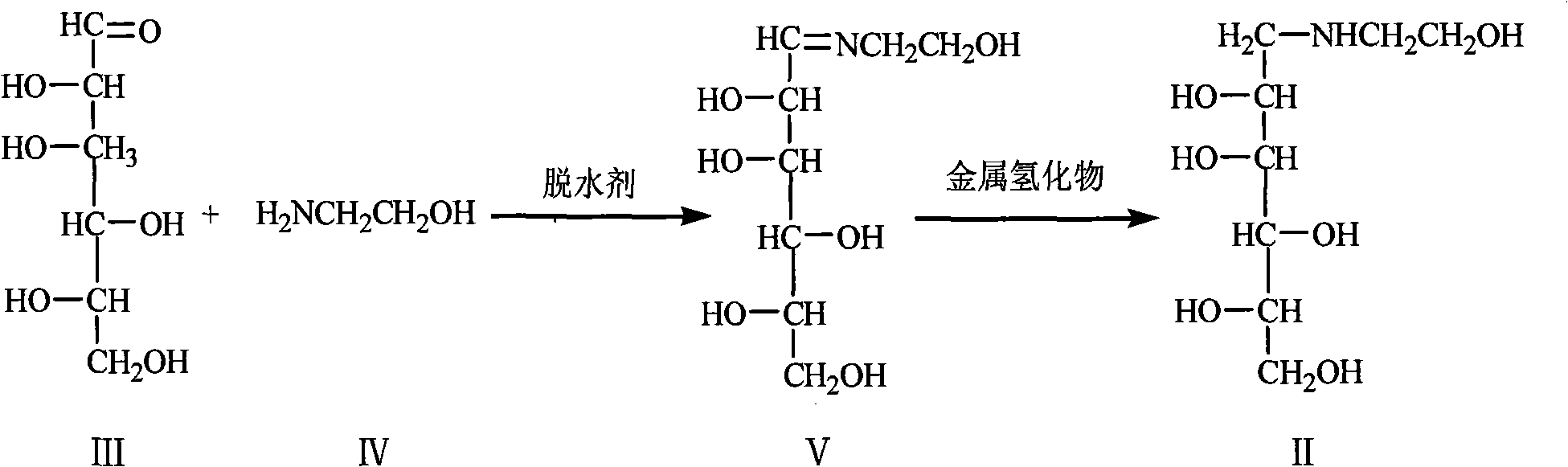
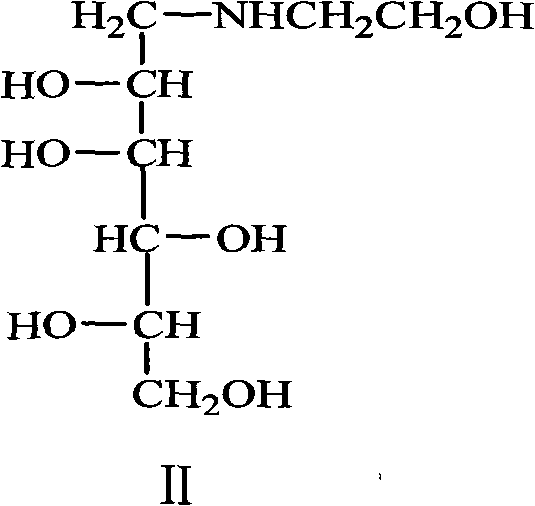Method for preparing miglitol intermediate N-hydroxyethyl glucosamine
A technology of glucosamine and miglitol, which is applied in the field of drug synthesis, can solve the problems of many by-products, difficult to purify, complicated steps and the like, and achieves the effects of safe reaction, low cost and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 1100ml of anhydrous methanol, 550g of glucose, and 201.5g of ethanolamine into a 5L four-neck flask, add 65g of anhydrous sodium carbonate, heat up to 52°C, react for 1 hour, cool down to 9°C, and slowly add 174.2g of lithium aluminum hydride, After about 5 hours, the reaction was completed, filtered, and 440 ml of ethyl acetate was added to the filtrate for crystallization. After 6 hours, suction filtered and dried to obtain the target product N-hydroxyethylglucamine.
[0030] Dissolve the crude product of N-hydroxyethylglucosamine in 495ml of methanol, add 9.9g of activated carbon, reflux for 1 hour, filter out the activated carbon, cool the filtrate to 12°C, add 396ml of ethyl acetate, crystallize, and suction filter after 7 hours. The filter cake was vacuum-dried for 24 hours (the temperature was 25-45° C., and the vacuum degree was greater than 0.09 MPa), and 661 grams of white N-hydroxyethylglucamine crystals were obtained, with a yield of 96%, a purity of 99% ...
Embodiment 2
[0032] Add 1100ml of absolute ethanol, 550g of glucose, and 205g of ethanolamine into a 5L four-necked bottle, add 97.2g of anhydrous sodium carbonate, heat up to 48°C, react for 0.5 hours, cool down to 8°C, slowly add 232.0g of lithium aluminum hydride, After about 6 hours, the reaction was completed, filtered, and 660 ml of ethyl acetate was added to the filtrate for crystallization. After 6 hours, suction filtered and dried to obtain the target product N-hydroxyethylglucamine.
[0033] Dissolve the crude product of N-hydroxyethylglucosamine in 500ml of methanol, add 50g of activated carbon, reflux for 1 hour, filter out the activated carbon, cool the filtrate to 8°C, add 500ml of ethyl acetate, crystallize, after 5 hours, filter with suction, The filter cake was vacuum-dried for 24 hours (at a temperature of 25-45° C. and a vacuum greater than 0.09 MPa) to obtain 675 grams of white N-hydroxyethylglucosamine crystals, with a yield of 98%, a purity of 99.5% (by HPLC detection)...
Embodiment 3
[0035] Add 825ml of n-propanol, 550g of glucose, and 203.5g of ethanolamine into a 5L four-necked bottle, add 35g of anhydrous calcium oxide, heat up to 51°C, react for 1.5 hours, cool down to 10°C, and slowly add 174.2g of sodium borohydride , about 7 hours, the reaction was completed, filtered, 600ml of ethyl acetate was added to the filtrate, crystallized, and after 8 hours, suction filtered and dried to obtain the target product N-hydroxyethylglucamine.
[0036] Dissolve the crude product of N-hydroxyethylglucosamine in 520ml of methanol, add 20.8g of activated carbon, reflux for 1 hour, filter out the activated carbon, cool the filtrate to 10°C, add 350ml of ethyl acetate, crystallize, after 6 hours, filter with suction, The filter cake was vacuum-dried for 24 hours (the temperature was 25-45° C., and the vacuum degree was greater than 0.09 MPa), and 647 grams of white N-hydroxyethylglucamine crystals were obtained, with a yield of 94%, a purity of 99% (detected by HPLC), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com