Hydroxyl telechelic polyester material based on piperazine block and preparation method thereof
A technology of hydroxyl-terminated polyester and piperazine, which is applied in the field of piperazine-based block hydroxyl-terminated polyester materials and their preparation, can solve the problem that the properties of polyester materials have little influence, the initiator has no biological activity, and the co-initiating In order to achieve the effect of controllable molecular weight, slowing down the effect of acid-induced autocatalytic degradation, good biocompatibility and biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
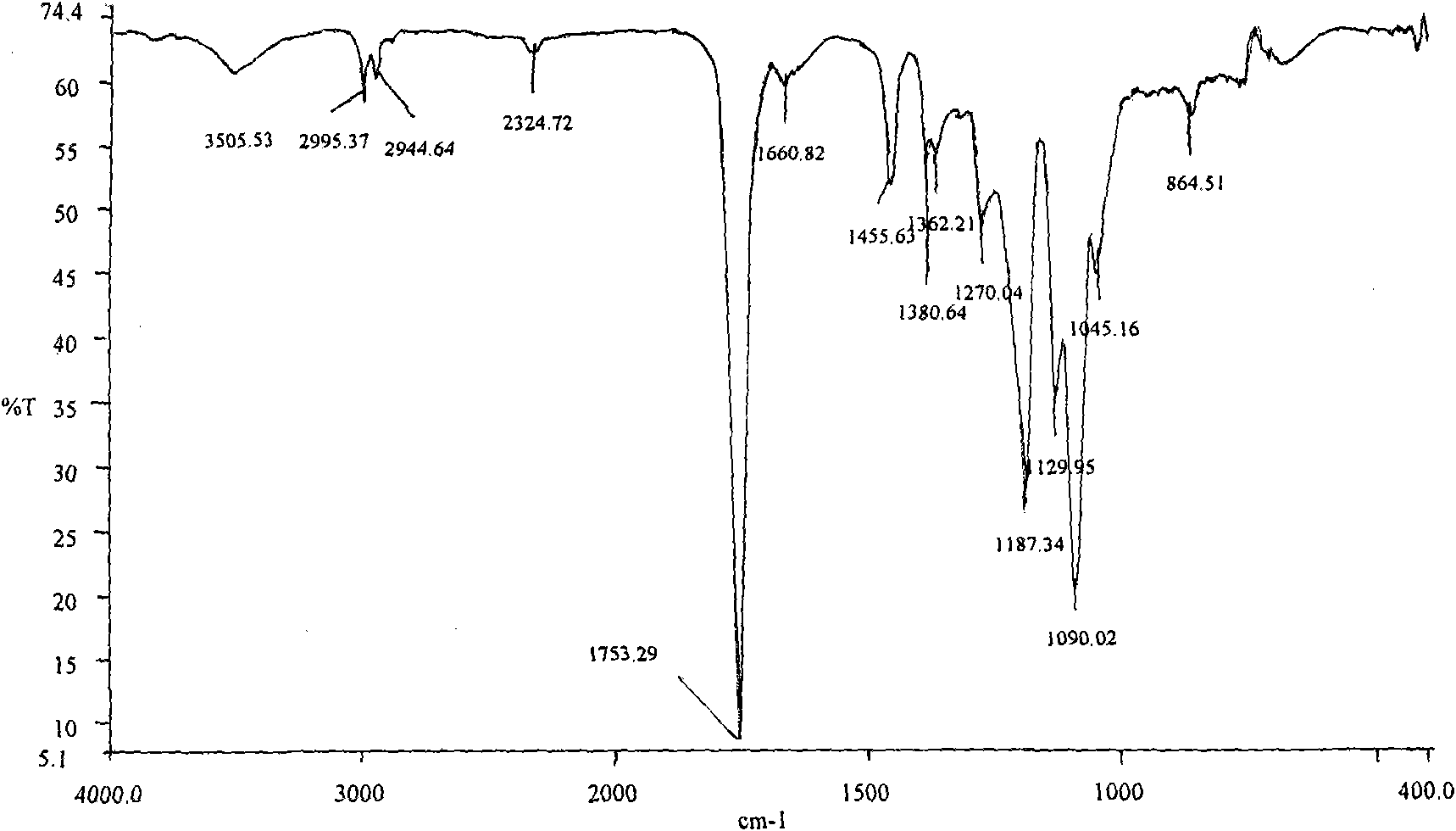
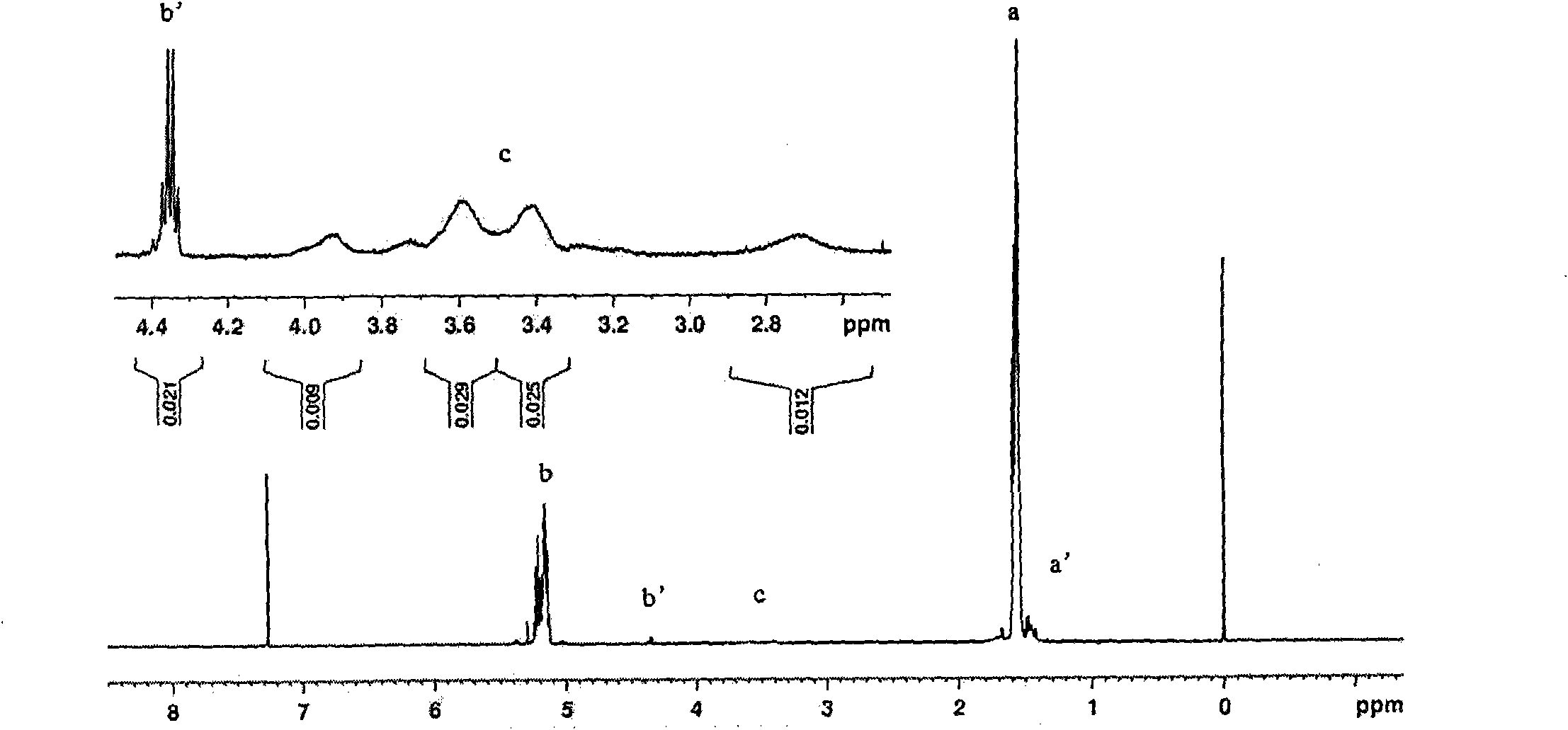
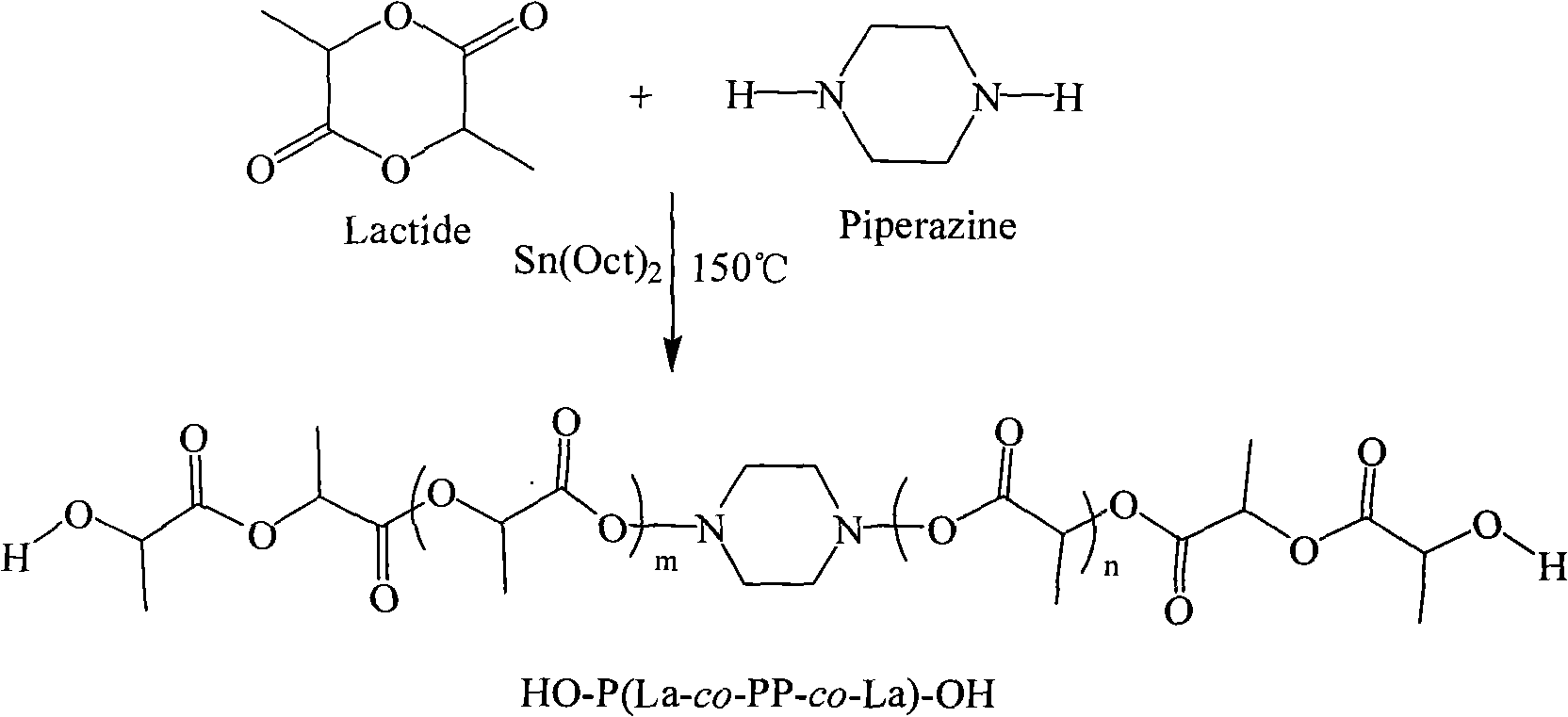
[0023] Weigh 5.000 g (0.03472 mol, molecular weight: 144) of D, L-lactide that has been distilled and recrystallized three times from ethyl acetate, and 0.5979 g (0.5979 g) of anhydrous piperazine ( 0.00694mol, molecular weight: 86.1), were added to a 25ml round bottom flask, and stannous octoate was added as an initiator according to 1 / 5000 of the molar weight of D, L-lactide, and then vacuumed 2 to 3 times, respectively After 15 minutes, seal the flask, place it in an oil bath to heat, control the temperature at 150°C, and react for 24 hours. After the reaction product was dissolved in dichloromethane, it was purified with n-hexane and dried at room temperature to obtain 3.512g polylactic acid with hydroxypiperazine block at the end, glass transition temperature T g =13.14°C, its molecular weight was determined to be 830 (theoretical molecular weight was 144×5+86.1=805.1) by the hydroxyl end titration analysis method.
[0024] The synthetic route of embodiment 1 is as follo...
Embodiment 2
[0027] Weigh 5.000 g (0.03472 mol, molecular weight: 144) of D, L-lactide that has been distilled and recrystallized three times from ethyl acetate, and 0.2988 g (0.2988 g) of anhydrous piperazine ( 0.00347mol, molecular weight: 86.1), were added to a 25ml round bottom flask, according to 1 / 5000 of the molar weight of D, L-lactide, stannous octoate was added as an initiator, and then vacuumed 2 to 3 times, respectively After 15 minutes, seal the flask, place it in an oil bath to heat, control the temperature at 150°C, and react for 24 hours. After the reaction product was dissolved in dichloromethane, it was purified with n-hexane and dried at room temperature to obtain 3.823g polylactic acid with hydroxypiperazine block, glass transition temperature T g =20.54°C, its molecular weight was determined to be 1132 (theoretical molecular weight was 144×10+86.1=1526.1) as determined by terminal hydroxyl titration analysis.
Embodiment 3
[0029] Weigh 5.000 g (0.03472 mol, molecular weight: 144) of D, L-lactide that has been distilled and recrystallized three times from ethyl acetate, and 0.1495 g (0.1495 g) of anhydrous piperazine ( 0.00173mol, molecular weight: 86.1), respectively added to a 25ml round bottom flask, added stannous octoate as an initiator according to 1 / 5000 of the molar weight of D, L-lactide, and then evacuated 2 to 3 times, respectively After 15 minutes, seal the flask, place it in an oil bath to heat, control the temperature at 150°C, and react for 24 hours. After the reaction product was dissolved in dichloromethane, it was purified with n-hexane and dried at room temperature to obtain 3.901 g of polylactic acid with a hydroxypiperazine block, with a glass transition temperature of T g =28.45°C, its molecular weight was determined to be 2576 (theoretical molecular weight was 144×20+86.1=2966.1) as determined by terminal hydroxyl titration analysis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com