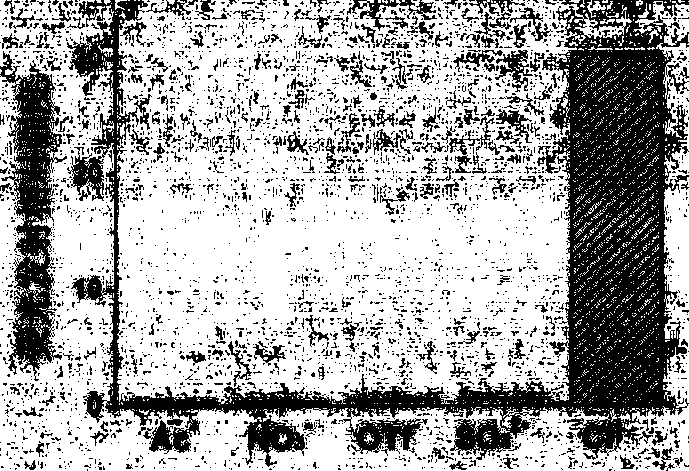
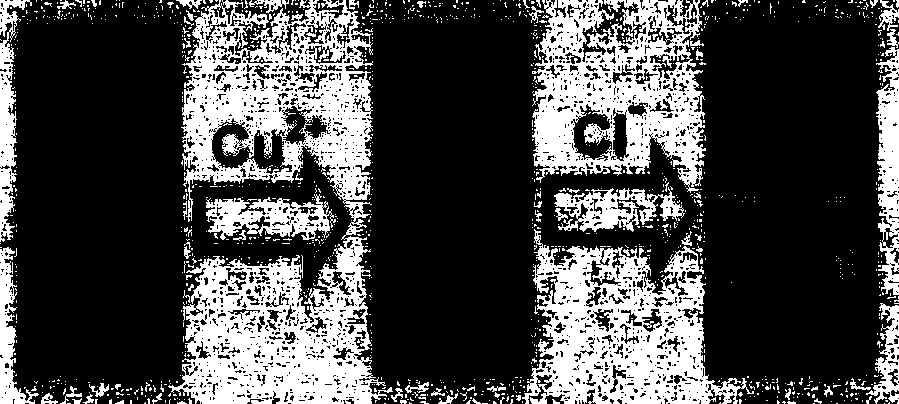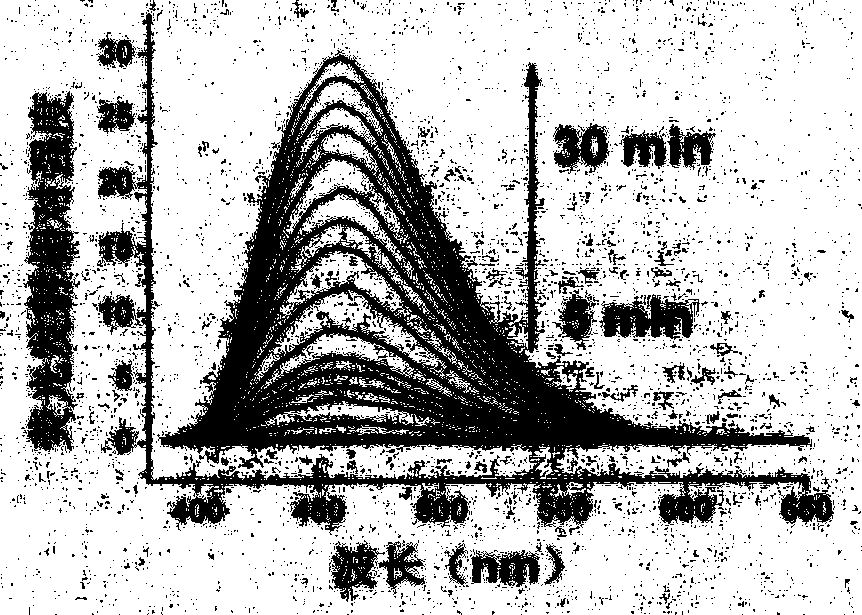Chloride ion fluorescent probe and preparation method and application thereof
A technology of fluorescent probes and chloride ions, which is applied in the field of ion detection in chemical analysis, can solve problems such as limiting anion types and fluorescence response sensitivity, and achieve the effects of convenient identification, good ion selectivity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] Preparation formula of the present invention:
[0037]
Embodiment 1
[0039]Under nitrogen protection, 100mg (0.24mmol) 2,7-dibromo-9-cycloheptatrienylfluorene, 106mg (0.53mmol) 2-biphenylboronic acid, 0.47g (3.4mmol) potassium carbonate, then add the mixed solvent of toluene (8ml), ethanol (8ml), water (8ml) in the ratio of 1:1:1 with the volume ratio, and add the tetrakis (triphenylene) of catalytic amount Phosphine) palladium, heated to reflux, reacted for 6 hours, cooled, extracted the reaction solution with dichloromethane, combined the organic layers, washed with saturated brine, dried the organic phase over magnesium sulfate, filtered, evaporated the solvent, and separated by column chromatography to obtain 2 , 7-bis(biphenyl-2-yl)-9-cycloheptatrienylene fluorene 42 mg, yield 31%.
Embodiment 2
[0041] Under nitrogen protection, 100mg (0.24mmol) 2,7-dibromo-9-cycloheptatrienylfluorene, 144mg (0.73mmol) 2-biphenylboronic acid, 0.47g (3.4mmol) potassium carbonate, then add the mixed solvent of toluene (8ml), ethanol (8ml), water (8ml) in the ratio of 1:1:1 with the volume ratio, and add the tetrakis (triphenylene) of catalytic amount Phosphine) palladium, heated to reflux, reacted for 12 hours, cooled, extracted the reaction solution with dichloromethane, combined the organic layers, washed with saturated brine, dried the organic phase with magnesium sulfate, filtered, evaporated the solvent, and separated by column chromatography to obtain 2 , 55 mg of 7-bis(biphenyl-2-yl)-9-cycloheptatrienylene fluorene, yield 41%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com