Preparation of new apolipoprotein C-I
Apolipoprotein, C-I technology, applied in the field of purification and identification, protein production, to achieve the effect of stable expression system, low production cost, and stable strain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Cloning and amplification of human apolipoprotein C-I gene (hApo C-I)
[0041] (1) Trizol method to extract total RNA from human liver tissue
[0042] The freshly isolated human liver tissue was cut into 100 mg size and immediately frozen in liquid nitrogen. Total RNA was extracted from tissues as follows. Take out 300 mg of frozen tissue and put it into a mortar filled with liquid nitrogen, and crush the tissue. Transfer the crushed tissue into a 50ml centrifuge tube, add about 5ml Trizol, and homogenize with a homogenizer at high speed for 15-30s at room temperature. Add 1.0ml of chloroform (200μl / ml Trizol), vortex fully, and place at room temperature for 5min. After centrifugation (12000r / min) at 4°C for 15min, the upper aqueous phase was transferred to another centrifuge tube. Add an equal volume of isopropanol, shake well, precipitate at room temperature for 10 minutes, centrifuge at 4°C (12000r / min) for 15 minutes, and discard the supernatant. ...
Embodiment 2
[0078] Example 2: Construction of hApoC-I Pichia secretory expression vector pPICZa / hApoC-I and host cell transformation
[0079] Take 50ng of the correct plasmid carrying the hApoC-I gene identified by the above sequencing, and set up a PCR reaction system in a 0.2ml EP tube according to the above ratio. Using the above PCR reaction system and conditions, with the help of upstream primer 5'-ATACTCGAGAAGAGAACCCCAGACGTCTCCA-3' (SEQ ID NO: 3) to introduce the partial sequence of yeast a factor signal peptide and XhoI site, and using downstream primer: 5'-GCCGAATTCTCATGAGTCAATCTTGAGTTTCTCC-3 ' (SEQ ID NO: 4) introduces an EcoRI site. After introducing the above sequences and restriction sites by PCR, recover the target fragment. After digestion with the corresponding enzymes, the plasmid pPICZa after digestion with the same enzymes was connected to construct the expression vector pPICZa / hApoC-I of Pichia pastoris, and then transformed into Escherichia coli, the plasmid was extra...
Embodiment 3
[0083] Embodiment 3: Expression and purification of ApoA-II polypeptide
[0084] (1) Expression of recombinant human apolipoprotein C-I (rhApoC-I)
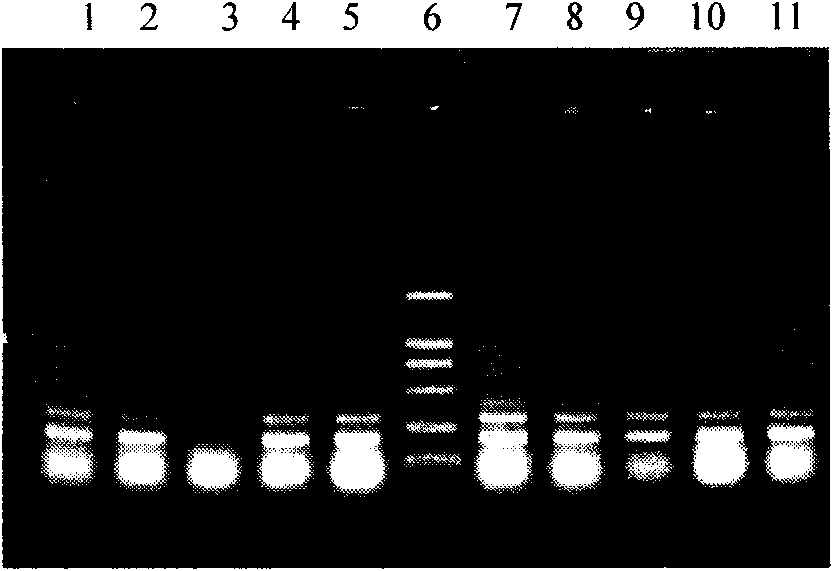
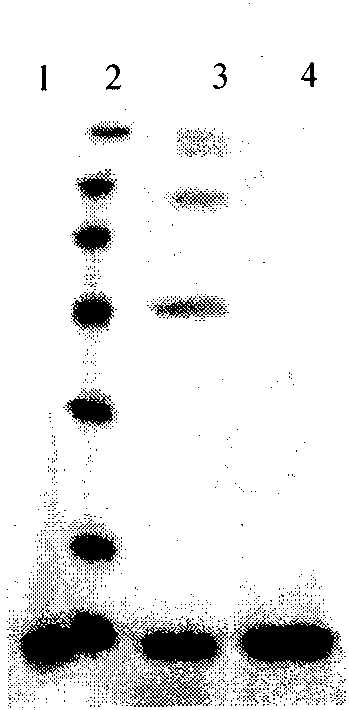
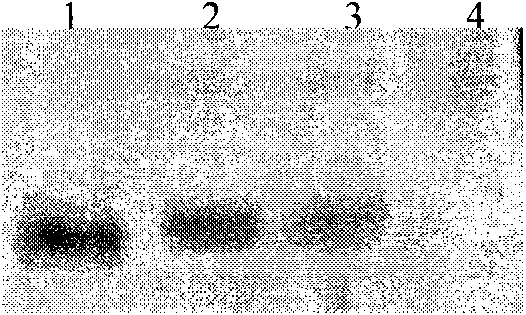
[0085] Inoculate the positive clones of the above identification results in 10ml of BMGY (pH6.0) medium, culture with shaking at 30°C for 24 hours, until OD 600 Cells were collected when reaching 2.0-6.0. Resuspend the cell pellet with an equal volume (10ml) of BMMY (pH6.0), culture with shaking at 30°C, and induce expression. During the induction process, methanol was supplemented every 24 hours to a final concentration of 0.5%, and sterilized ultrapure water was supplemented at the same time to keep the total volume of the fermentation broth constant. At the 0, 24, 48, 72, 96, 120, 144, and 168 hours of culture, 0.5 ml of fermentation broth was taken, and the supernatant was centrifuged for protein analysis (SDS-PAGE analysis, Western Blot analysis).
[0086] (2) Purification of rhApoC-I
[0087] The fermentation broth is co...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap