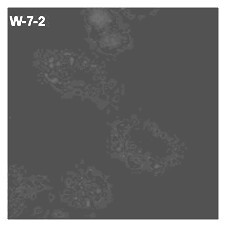
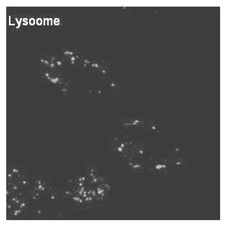
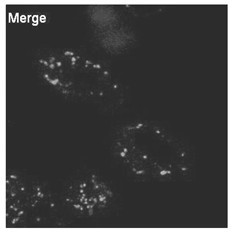
Lysosome targeted fluorescent substance and synthesis method thereof
A technology of fluorescent substances and lysosomes, applied in the fields of biochemical equipment and methods, fluorescence/phosphorescence, chemical instruments and methods, etc., can solve the problems of small scope, easily inactivated antibodies, high price, etc., and achieve stable product properties, Simple synthetic route and high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis of intermediate 5,11-dibromo-[1,5-diazaocine]diazaoctene tetraene:
[0026] 6.9g of 4-bromoaniline and 2.4g of paraformaldehyde (n=30) were mixed and placed in a 250mL long-necked flask, a magnetic stirrer was added, and the reaction system was cooled to -20°C in a cold trap. Under the condition of magnetic stirring, 80 mL of trifluoroacetic acid was slowly added dropwise to the system with a 100 mL constant pressure dropping funnel, and the trifluoroacetic acid was completely added dropwise in about 50 minutes. After the 4-bromoaniline and paraformaldehyde in the reaction system were completely dissolved, the temperature of the system was raised to 0° C., and the reaction was continued for 140 hours. After finishing the reaction, dissolve the solid solution with sodium hydroxide (NaOH) to form a 1mol / L solution. The reacted solution was slowly added dropwise into the sodium hydroxide solution while stirring, and a large amount of solids were preci...
Embodiment 2
[0027] Example 2: Synthesis of intermediate 5,11-dibromo-[1,5-diazaocine]diazacyclooctene tetraene
[0028] 6.9g of 4-bromoaniline and 3.0g of paraformaldehyde (n=30) were mixed and placed in a 250mL long-necked flask, and a magnetic stirrer was added to react at room temperature. Under the condition of magnetic stirring, 80 mL of trifluoroacetic acid was slowly added dropwise to the system with a 100 mL constant pressure dropping funnel, and the trifluoroacetic acid was completely added dropwise in about 50 minutes. 4-bromoaniline and paraformaldehyde in the reaction system were completely dissolved, and the reaction was continued for 140 hours. After finishing the reaction, dissolve the solid solution with sodium hydroxide (NaOH) to form a 1mol / L solution. The reacted solution was slowly added dropwise into the sodium hydroxide solution while stirring, and a large amount of solids were precipitated. After the dropwise addition, the pH of the solution was about 8, and it wa...
Embodiment 3
[0029] 6.9 g of 4-bromoaniline and 2.4 g of paraformaldehyde (n=30) were mixed and placed in a 250 mL long-necked flask, and a magnetic stirring bar was added. Under the condition of -20°C and magnetic stirring, 80 mL of trifluoroacetic acid was slowly added dropwise to the system with a 100 mL constant pressure dropping funnel, and the trifluoroacetic acid was completely added dropwise in about 50 minutes. The 4-bromoaniline and paraformaldehyde in the reaction system were completely dissolved, and the reaction was continued at 0° C. for 70 hours. After finishing the reaction, dissolve the solid solution with sodium hydroxide (NaOH) to form a 1mol / L solution. The reacted solution was slowly added dropwise into the sodium hydroxide solution while stirring, and a large amount of solids were precipitated. After the dropwise addition, the pH of the solution was about 7, extracted with 100mL of chloroform, extracted continuously for 3 times, and combined the organic phases. Rota...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com