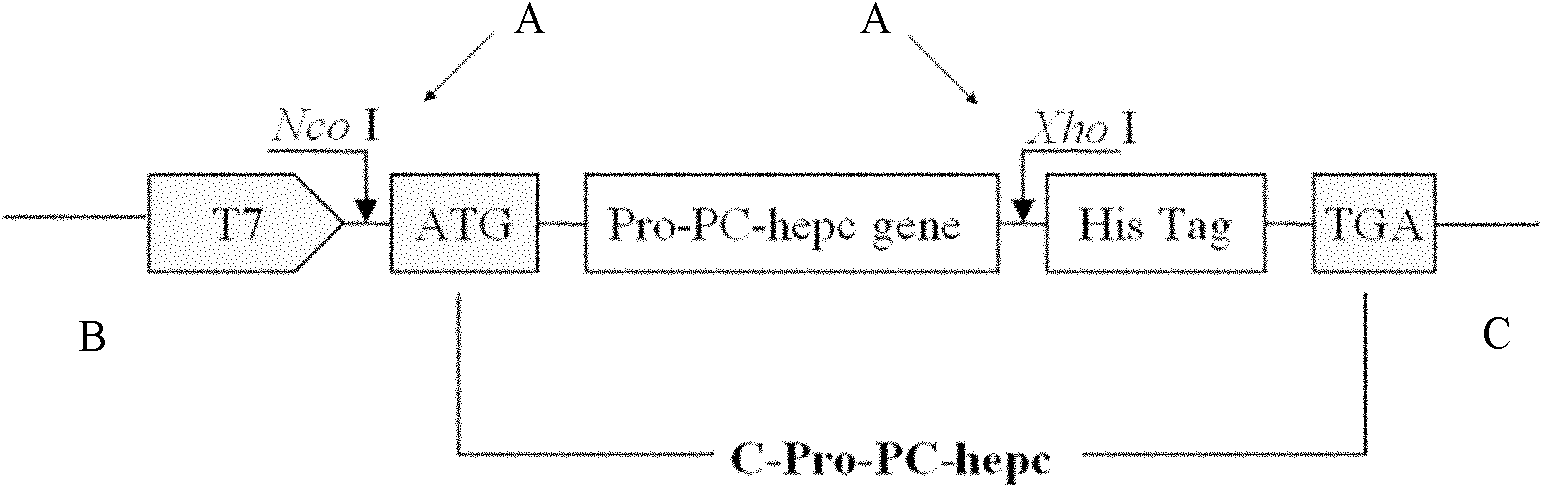
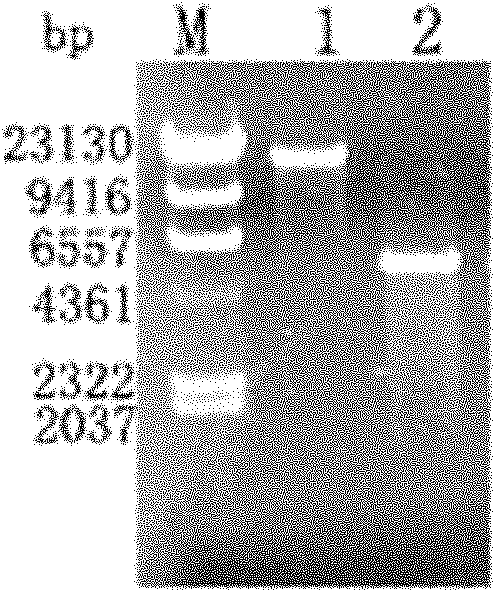Large yellow croaker hepcidin antibacterial peptide and preparation method thereof
A large yellow croaker and antimicrobial peptide technology, applied in the field of fish genetic engineering, can solve the problems of antimicrobial peptides without broad-spectrum antibacterial effect, high cost, complex structure, etc., and achieve the effect of increasing the molecular weight of the expression product, low price, and simple configuration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1pET-28a + Carrier handling
[0053] 1)pET-28a + Plasmid extraction;
[0054] Will contain pET-28a + The E.coli BL21(DE3)pLysS of the prokaryotic expression vector was stored in 20% glycerol and streaked on an LB plate containing 50μg / ml kanamycin and cultured at 37°C for 12h. Pick a single clone, inoculate it in 5ml of LB liquid medium supplemented with 50μg / ml kanamycin and 0.5% D-glucose, culture at 37°C, 180rpm for 8h, and extract the plasmid according to the instructions of the Dongsheng Plasmid Minimal Extraction Kit.
[0055] 2)pET-28a + Enzyme digestion and dephosphorylation treatment of the vector;
[0056] According to the following system for pET-28a + The plasmid is digested with NcoI-XhoI
[0057] NcoI-XhoI double digestion:
[0058] 10×K Buffer (buffer) 15μl
[0059] 0.1% BSA 15μl
[0060] NcoI endonuclease 7μl
[0061] XhoI endonuclease 7μl
[0062] pET-28a + Plasmid 100μl
[0063] Ultrapure water 6μl
[0064] Total volume 150μl
[0065] After incubating at 37°C f...
Embodiment 2
[0071] Example 2 Obtaining the target gene
[0072] 1) Design of primers:
[0073] According to the multiple cloning sites on the pET-28a+ vector and the large yellow croaker hepcidin cDNA sequence, an expression vector containing the leader peptide of the large yellow croaker hepcidin antimicrobial peptide was designed (see figure 2 ), PCR upstream and downstream primers are shown in Table 1, boldface indicates restriction enzyme sites, C-Pro-PC-hepc forward, where CCATGG is the Nco I restriction site, and the front GCG is the protection base of the restriction site , C-Pro-PC-hepc reverse where CTCGAG is the restriction site of Xho I, and the front CCG is the designed protective base:
[0074] Table 1. Upstream and downstream primers used to express C-Pro-PC-hepc
[0075]
[0076] 2) PCR amplification of hepcidin antimicrobial peptide gene sequence of large yellow croaker
[0077] Use the pPMD18-T positive plasmid containing the cDNA of large yellow croaker hepcidin as a template, a...
Embodiment 3
[0106] Example 3 Construction, transformation and identification of expression vector
[0107] Connect the treated pET-28a+ prokaryotic expression vector with the target fragment of the large yellow croaker hepcidin antimicrobial peptide cDNA containing the same sticky end. The reaction system is as follows:
[0108] pET-28a+ 2μl
[0109] Target fragment 1μl
[0110] Ultrapure water 1.8μl
[0111] T4 DNA Ligase 0.2μl
[0112] Ligation Solution I 5μl
[0113] Total volume 10μl
[0114] Ligation overnight at 16°C, the next day, take 5μl of the ligation reaction solution and transform it into 50μl E.coli TOP10F' competent cells, spread it on an LB agar plate containing 50μg / mL kanamycin and 0.5% D-glucose and incubate at 37°C overnight. A single colony was picked the next day, and positive clones were picked, cultured at 37°C and 180 rpm for 8 hours, and plasmids were extracted according to the instructions of the Dongsheng Plasmid Minimal Extraction Kit. Use the upstream and downstream pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com