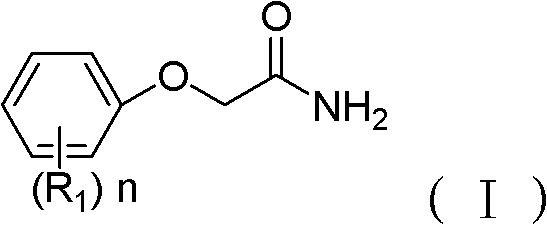
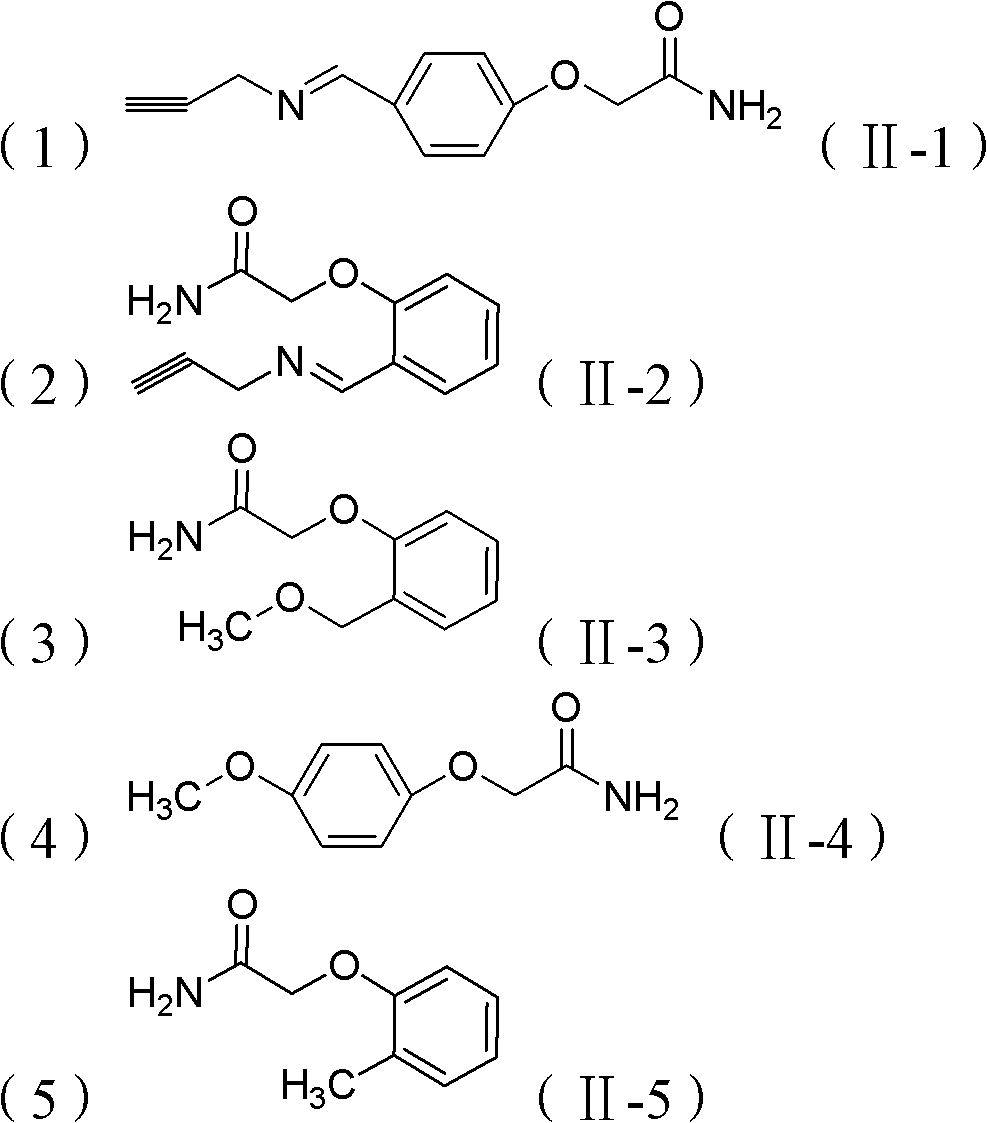
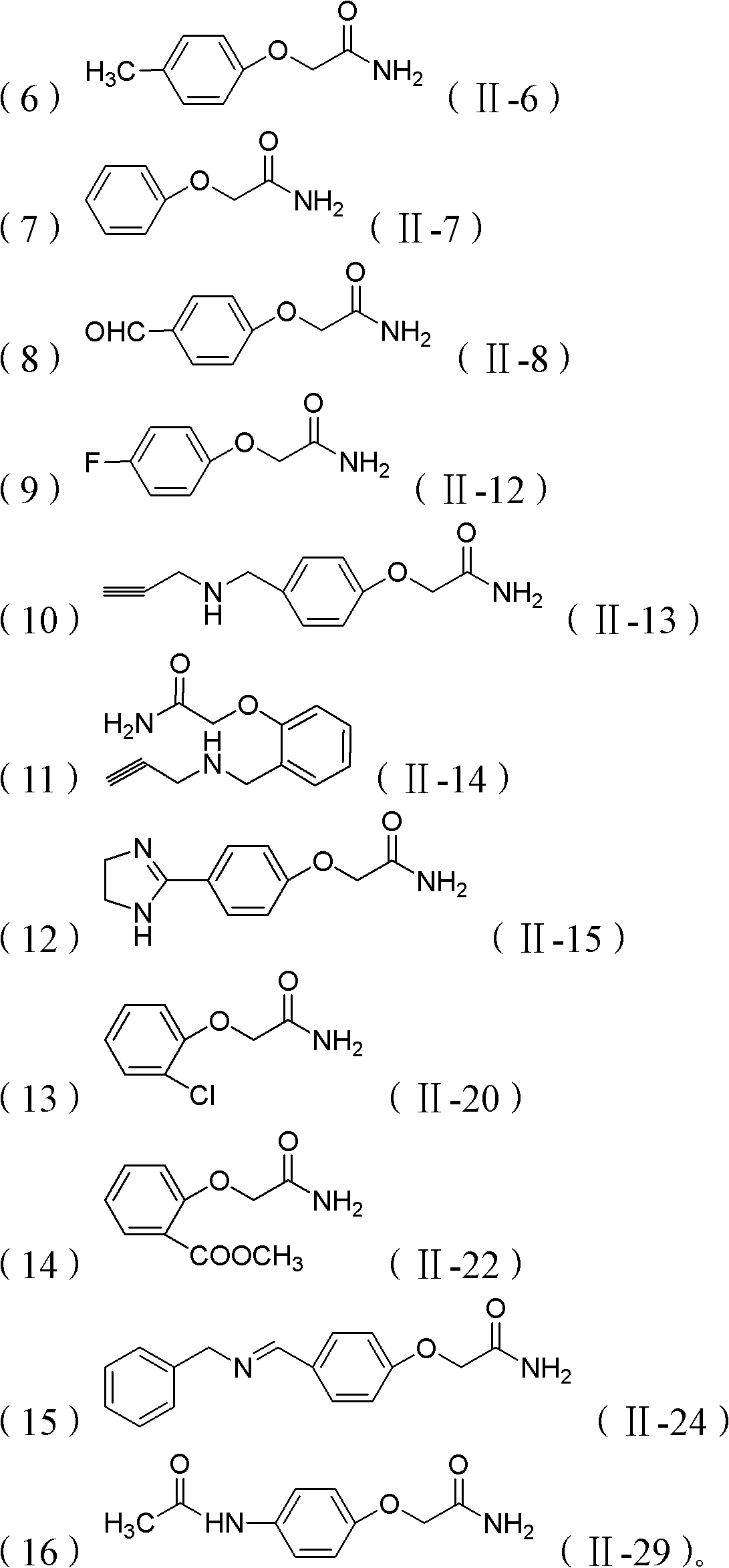
Amide compound and application thereof in preparing monoamine oxidase inhibitor
A technology of amide compounds and monoamine oxidase, which is applied in organic chemistry, drug combination, medical preparations containing active ingredients, etc., can solve the problems of psychosis, side effects, liver toxicity, etc. The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Reaction formula:
[0035]
[0036] Method:
[0037] (1) Dissolving the phenolic compound (II) in DMF solvent, then adding NaOH and 2-chloroacetamide, and heating at 85°C for about 6 hours. The amount of phenolic compound: 2-chloroacetamide: NaOH is 5mmol: 5mmol: 7.2mmol, and the volume of DMF is 10ml.
[0038] (2) Dilute the amide compound constructed in step (1) with DMSO solvent to a concentration of 100 μM, pipette 4 μL of the diluent and add it to the reaction well of a 96-well plate, then add 400 μL of pH8.4 boric acid buffer and 4 μL of BSA, and add MAO -A (20mg / mL) or MAO-B (20mg / mL) 4μL, the mixture was reacted at 38°C for 3h, and then the fluorescent probe 4-methyl-7-(3-aminopropyl)-coumarin 2μL ( 10mmol / mL), then react at 38°C for 2h, and use a fully functional spectrofluorometer (λ ex / λ em =365 / 460nm) for detection, judge the level of inhibitory activity of amide compounds on monoamine oxidase according to the level of fluorescence value: the stronge...
Embodiment 2
[0043]Embodiment 2: the preparation of 2-(4-((propene-2-imine) methyl) phenoxy) acetamide (II-1)
[0044]
[0045] The synthetic route is as follows:
[0046]
[0047] Add 4-hydroxybenzaldehyde (6mmol), NaOH (7.2mmol) and DMF (10ml) into a 50ml round-bottomed flask. After the dissolution is complete, add 2-chloroacetamide (5mmol), stir at 85°C for 6h, and the reaction is complete After 3*20ml dichloromethane extraction, NaHCO 3 Wash with saturated solution, dry over anhydrous magnesium sulfate, filter, drain, and purify by column chromatography to obtain a white solid powder with a yield of 91%. Dissolve the obtained white powder in dichloromethane, add one equivalent of propargyl amine to react for one hour, extract with 3*20ml dichloromethane, NaHCO 3 Wash with saturated solution, dry over anhydrous magnesium sulfate, filter, drain, and purify by column chromatography to obtain a white solid product with a yield of 85%. Compound Characterization: 1 H-NMR (CDCl 3 )...
Embodiment 3
[0048] Embodiment 3: Preparation of 2-(4-((propylene-2-imine)methyl)phenoxy)acetamide (II-2)
[0049]
[0050] The synthetic route is as follows:
[0051]
[0052] Add 2-hydroxybenzaldehyde (6mmol), NaOH (7.2mmol) and DMF (10ml) into a 50ml round-bottomed flask. After the dissolution is complete, add 2-chloroacetamide (5mmol), stir at 85°C for 6h, and the reaction is complete After 3*20ml dichloromethane extraction, NaHCO 3 Wash with saturated solution, dry over anhydrous magnesium sulfate, filter, drain, and purify by column chromatography to obtain a white solid powder with a yield of 88%. Dissolve the obtained white powder in dichloromethane, add one equivalent of propargyl amine to react for one hour, extract with 3*20ml dichloromethane, NaHCO 3 Wash with saturated solution, dry over anhydrous magnesium sulfate, filter, drain, and purify by column chromatography to obtain a white solid product with a yield of 74%. Compound Characterization: 1 H-NMR (CDCl 3 ): δ1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com