Solid preparation and preparation method thereof
A technology for solid preparations and adhesives, which is used in pharmaceutical formulations, medical preparations with inactive ingredients, and inorganic inactive ingredients. Wet stability of formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Embodiment 1 Nystatin vaginal effervescent tablet
[0101] Component mg / tablet
[0102] Nystatin (anhydrous substance) 33.3 (100,000u)
[0103] Isomalt GalenIQ 721 320
[0104] Polyethylene glycol 6000 96
[0105] Croscarmellose Sodium 17.7
[0106] Aspartan 1
[0107] Sodium bicarbonate 8.4
[0108] Monosodium citrate 19.6
[0109] Silica 4
[0110]
[0111] Total 500
[0112] Nystatin, Isomalt GalenIQ 721, polyethylene glycol 6000, and croscarmellose were fully dried (the residual moisture in the raw and auxiliary materials does not affect the stability of the preparation and the detection of moisture-unstable substances, the same below). Grind plain sodium, aspartame, sodium bicarbonate, monosodium citrate, and silicon dioxide separately, pass through a 60-mesh sieve, and mix uniformly by equal-volume incremental method. Pack 500 mg of the above-mentioned mixture into each vesicle in the punched packaging film m...
example 1
[0114] Reference Example 1: Replace the Isomalt GalenIQ 721 and two-thirds of the polyethylene glycol 6000 in the prescription with the same weight of α-lactose and microcrystalline cellulose, and prepare the reference product 1 by the above method.
example 2
[0115] Reference example 2: replace two-thirds of Isomalt GalenIQ 721 and four-fifths of polyethylene glycol 6000 in the prescription with α-lactose and microcrystalline cellulose of the same weight, and use the above method Preparation of reference product 2.
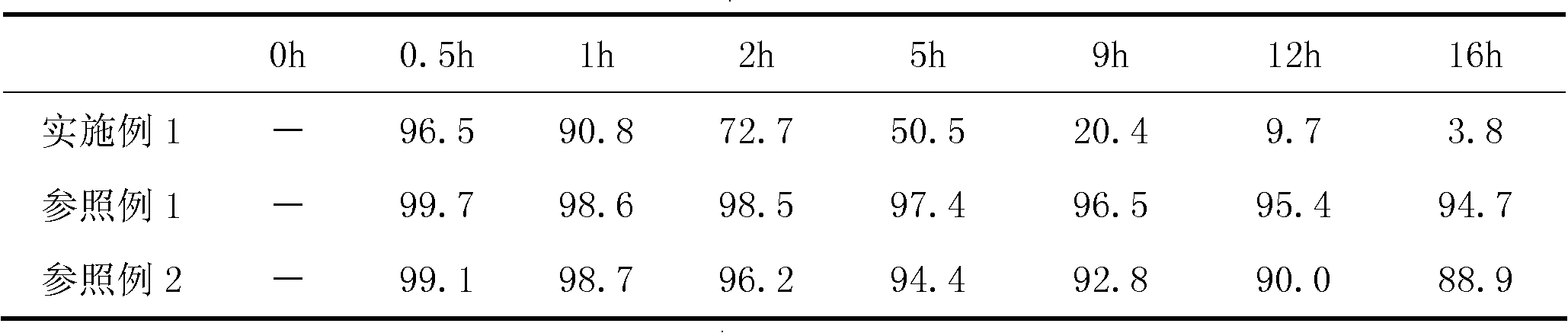
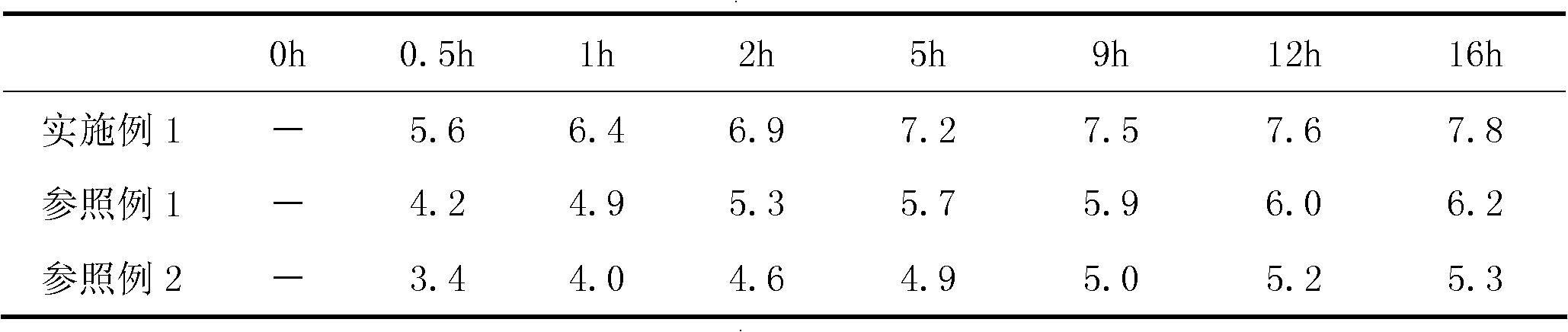
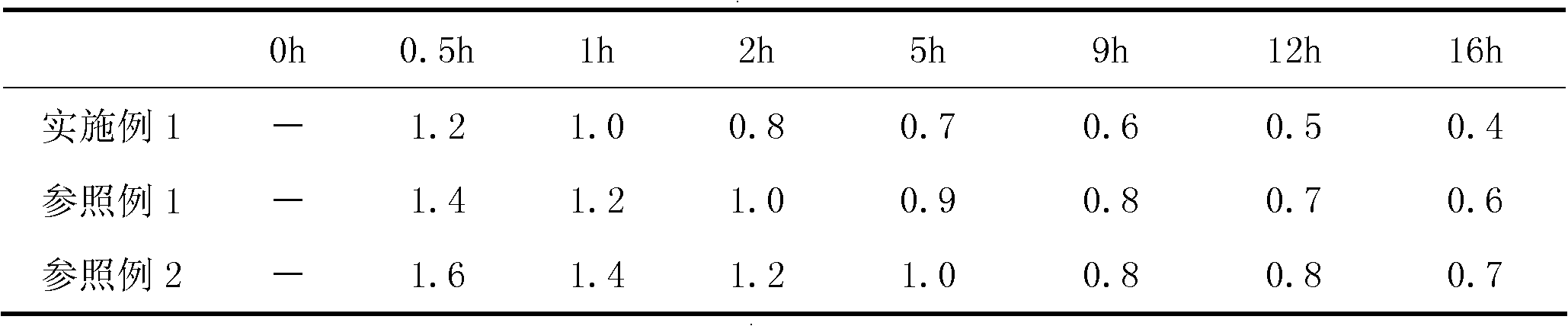
[0116] According to the method described in the instructions, detect the maximum change after the sodium bicarbonate in the above-mentioned embodiment and the reference example sampling sample react with monosodium citrate under the effect of moisture under the condition of relative humidity 75% and temperature 25 ℃ Amount (take a sample after a period of time during which the original content of the moisture-unstable substance therein can be reduced or changed by at least about 35% during the unhealed treatment, so that the sampling is repeated for a total of not less than 5 times, and the maximum amount of the moisture-labile substance The last 2 consecutive fluctuation range ratios of the amount of wet action variat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com