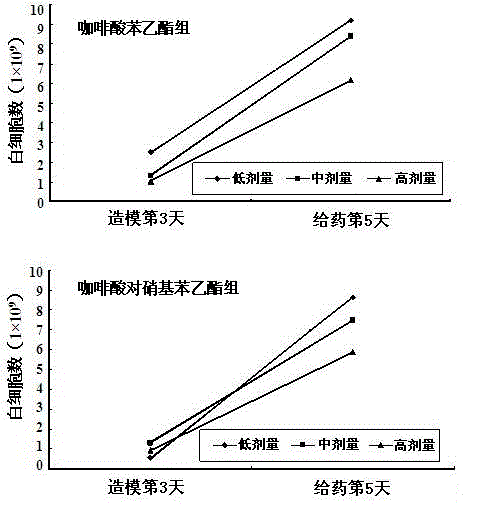
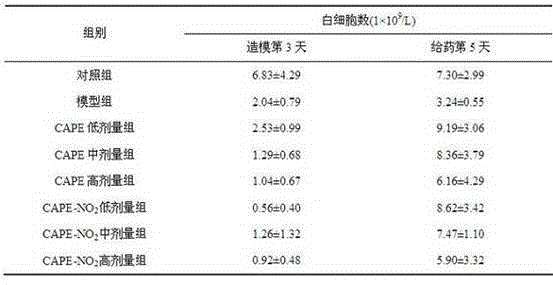
Caffeic acid p-nitrophenyl ethyl ester as well as preparation method and application of caffeic acid p-nitrophenyl ethyl ester
A technology of nitrophenethyl ester and caffeic acid, applied in the fields of chemistry and pharmacy, can solve the problems of uncertain curative effect, obvious side effects, high price and the like, and achieve the effects of increasing the number of peripheral blood white blood cells, good curative effect and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1, the preparation of p-nitrophenethyl caffeic acid (with 1,4-dioxane as solvent)
[0016] Put caffeic acid (1.02g, 5.6mmol) in a 50ml three-necked flask, add 25ml of 1,4-dioxane to dissolve, then add thionyl chloride (0.6ml, 8.2mmol), heat to 100°C for reflux reaction, Then 10 ml of a 1,4-dioxane solution of p-nitrophenylethanol (1.40 g, 8.4 mmol) was added dropwise, and the reflux reaction was continued, and the reaction progress was monitored by thin-layer chromatography. After the reaction was completed, the reaction liquid was naturally cooled, diluted with water, extracted 3 to 4 times with ethyl acetate, combined with the ethyl acetate extracts, dried overnight with anhydrous magnesium sulfate, filtered with suction, and the filtrate was distilled off under reduced pressure to obtain yellow Brown liquid, separated and purified by column chromatography (silica gel 200~300 mesh), using ethyl acetate-petroleum ether (volume ratio 1:2) mixed solvent as t...
Embodiment 2
[0017] Embodiment 2, the preparation of p-nitrophenethyl caffeic acid (with acetonitrile as solvent)
[0018] Put caffeic acid (1.02g, 5.6mmol) in a 50ml three-neck flask, add 25ml of acetonitrile to dissolve, then add thionyl chloride (0.6ml, 8.2mmol), heat to 80°C for reflux reaction, then add p-nitrobenzene dropwise Ethanol (1.40g, 8.4mmol) in acetonitrile solution 10ml, continue to reflux reaction, monitor the progress of the reaction with thin layer chromatography. After the reaction was completed, the reaction liquid was naturally cooled, diluted with water, extracted 3 to 4 times with ethyl acetate, combined with the ethyl acetate extracts, dried overnight with anhydrous magnesium sulfate, filtered with suction, and the filtrate was distilled off under reduced pressure to obtain yellow Brown liquid, separated and purified by column chromatography (silica gel 200~300 mesh), using ethyl acetate-petroleum ether (volume ratio 1:2) mixed solvent as the eluent, collecting t...
Embodiment 3
[0019] Embodiment 3, the preparation of p-nitrophenethyl caffeic acid (using chloroform as solvent)
[0020] Put caffeic acid (1.02g, 5.6mmol) in a 50ml three-necked flask, add 25ml of chloroform to dissolve, then add thionyl chloride (0.6ml, 8.2mmol), heat to 65°C for reflux reaction, then add p-nitrogen dropwise Trichloromethane solution 10ml of phenylethyl alcohol (1.40g, 8.4mmol), continue to reflux reaction, monitor reaction process with thin-layer chromatography. After the reaction was completed, the reaction liquid was naturally cooled, diluted with water, extracted 3 to 4 times with ethyl acetate, combined with the ethyl acetate extracts, dried overnight with anhydrous magnesium sulfate, filtered with suction, and the filtrate was distilled off under reduced pressure to obtain yellow Brown liquid, separated and purified by column chromatography (silica gel 200~300 mesh), using ethyl acetate-petroleum ether (volume ratio 1:2) mixed solvent as the eluent, collecting th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com