Preparation method of 5-aminobenzimidazole
A technology of aminobenzimidazole and nitrobenzimidazolone is applied in the field of preparation of 5-aminobenzimidazole, can solve the problems of troublesome post-processing, less equipment investment, large environmental pollution and the like, and achieves less side reactions and higher quality Good, high-yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
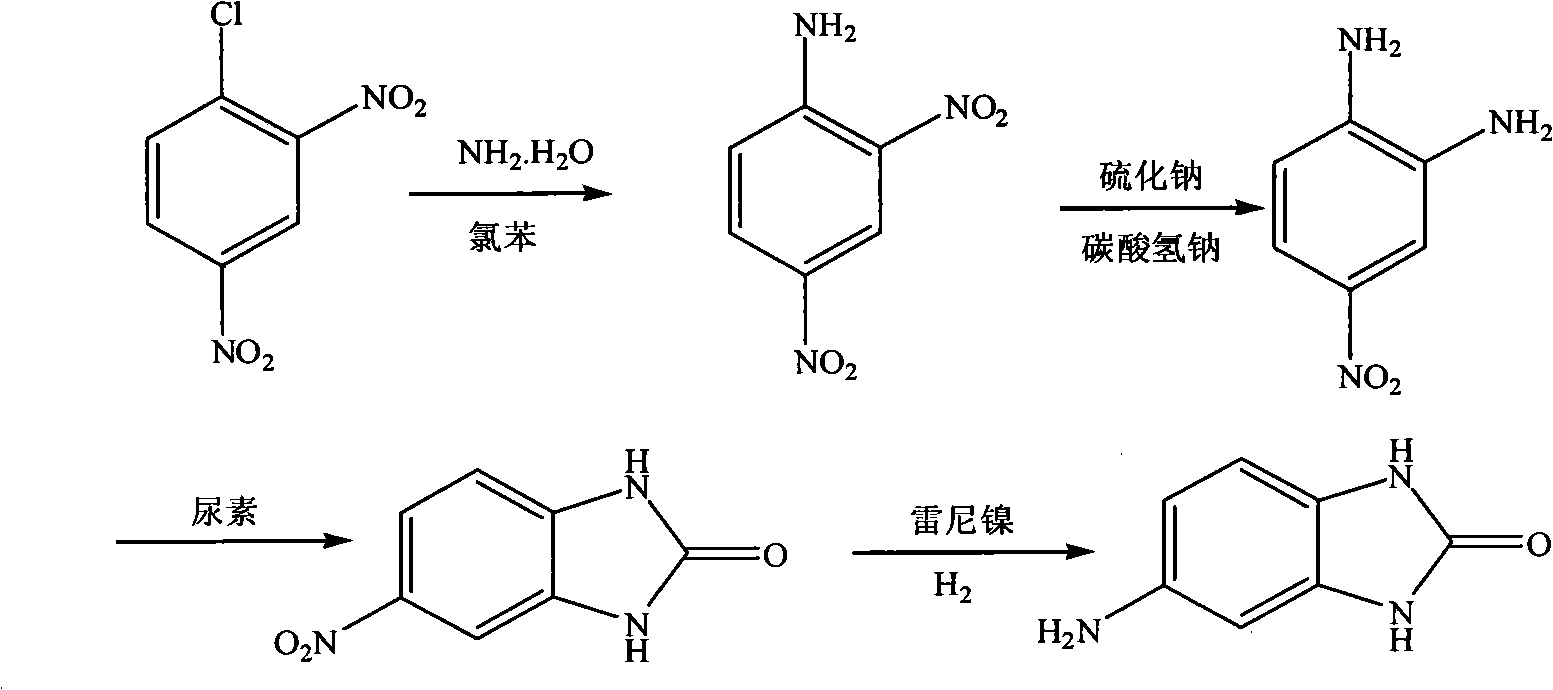
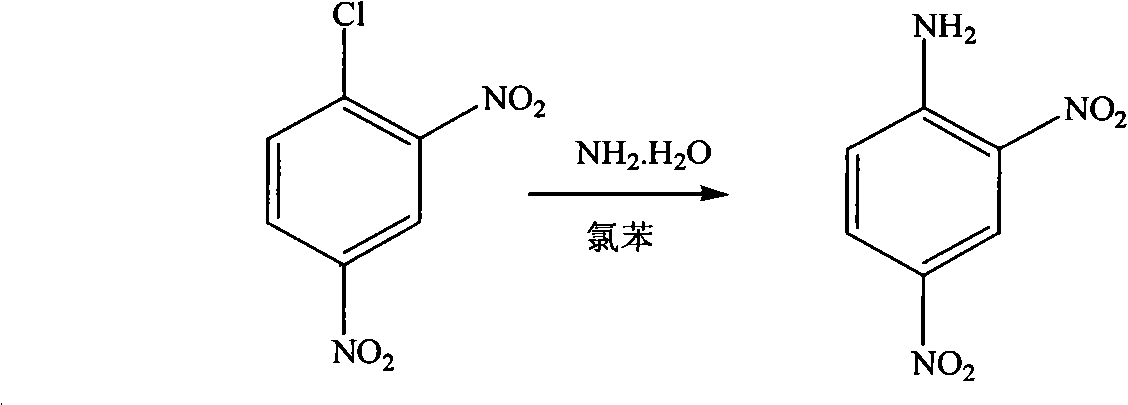
[0020] 1) Synthesis of 2,4-dinitroaniline:
[0021] In the autoclave, add 2,4-dinitrochlorobenzene (50.5g, 0.25mol), chlorobenzene (25mL) and 25-28wt.% ammonia water (50g), seal it, heat it to 145-150°C, and keep the temperature React at 145-150°C, 0.8-1.0Mpa for 1.5h, cool to room temperature, filter with suction, wash the filter cake with chlorobenzene (20mL) and water (50mL), recrystallize with ethanol, and dry to constant weight;
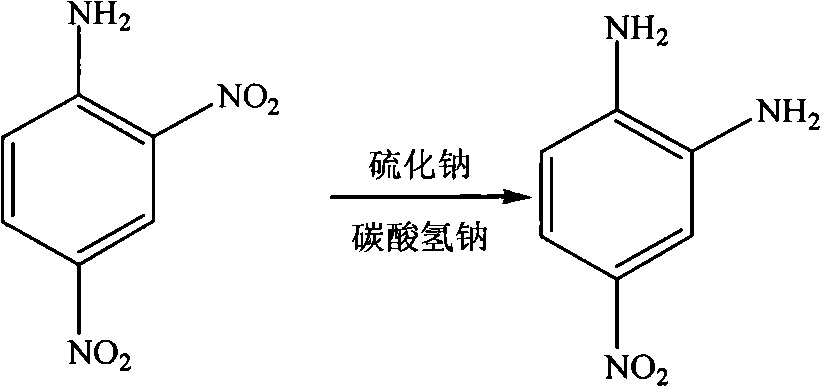
[0022] 2) Synthesis of 4-nitro-1,2-phenylenediamine:
[0023] In the reactor, add 2,4-dinitroaniline (42.1g, 0.23mol) and 35vol.% ethanol aqueous solution (500mL), stir, and heat to 65~70°C; at the same time, sodium sulfide nonahydrate (138.1g , 0.575mol) was dissolved in hot water (500mL) at 60°C, sodium bicarbonate (48.3g, 0.575mol) was added into the sodium sulfide solution and stirred to dissolve to obtain a mixed solution of sodium sulfide and sodium bicarbonate; sodium sulfide and Add the mixed solution of sodium bicarbonate dropwise int...
Embodiment 2
[0029] 1) Synthesis of 2,4-dinitroaniline:
[0030] In the autoclave, add 2,4-dinitrochlorobenzene (135.7g, 0.67mol), chlorobenzene (70mL) and 25-28wt.% ammonia water (400g), seal, heat to 150 ° C, and keep this temperature, 0.8~1.0Mpa, react for 1.5h, cool to room temperature, filter with suction, wash the filter cake with chlorobenzene (50mL) and water (50mL×2), recrystallize with ethanol, and dry to constant weight;
[0031] 2) Synthesis of 4-nitro-1,2-phenylenediamine:
[0032] In the reactor, add 2,4-dinitroaniline (115.4g, 0.63mol) and 50vol.% ethanol aqueous solution (1200mL), stir, and heat to 70°C; mol) was dissolved in water (3000mL) at 60°C, sodium bicarbonate (158.8g, 1.89mol) was added into the sodium sulfide solution and stirred to dissolve to obtain a mixed solution of sodium sulfide and sodium bicarbonate; sodium sulfide and sodium bicarbonate The mixed solution was added dropwise into the reactor, reacted at 65°C for 1h, cooled, diluted with water, filtered ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com