Polymer electrolyte and preparation method and application thereof
An electrolyte and polymer technology, applied in circuits, electrical components, secondary batteries, etc., can solve problems such as energy density bottlenecks of lithium-ion secondary batteries, hidden safety hazards of lithium-ion secondary batteries, internal short circuits of batteries, etc., and achieve high energy The effect of density, high toughness and high plasticity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, preparation No. 1 polymer electrolyte
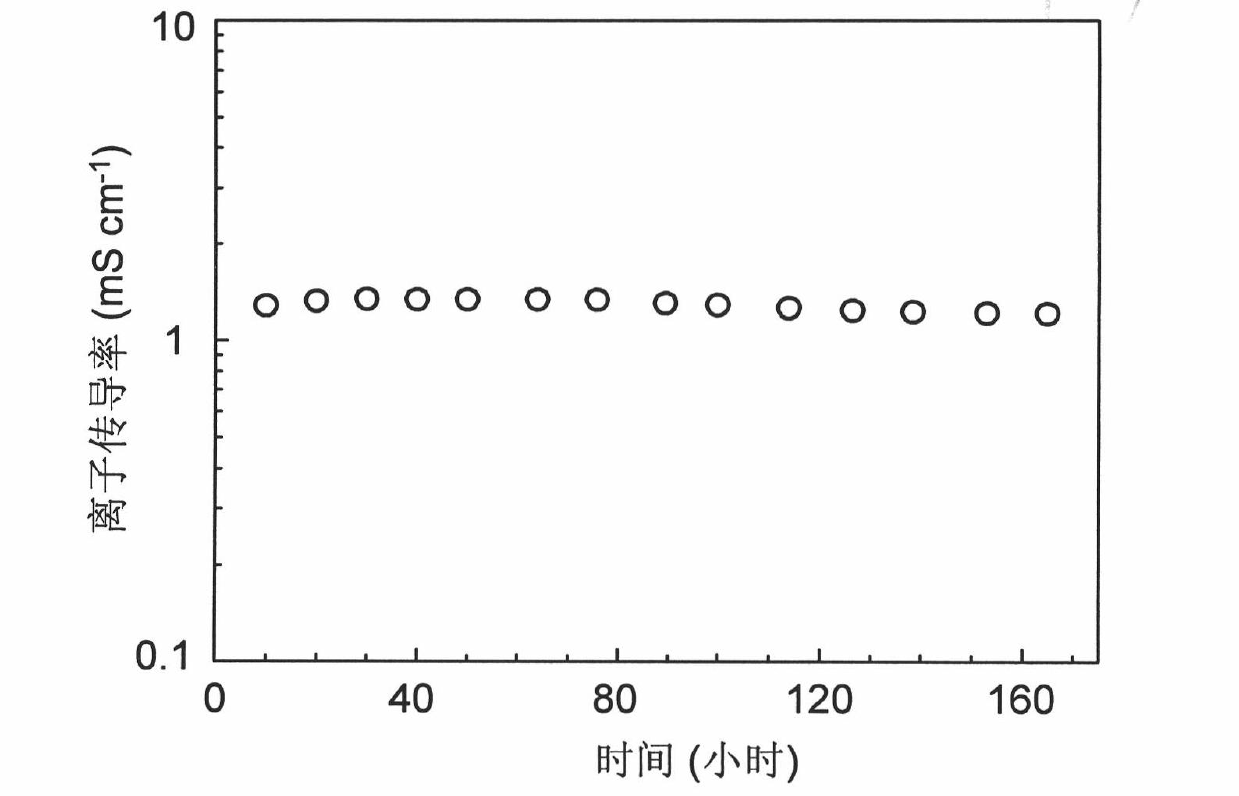
[0028] Under ultrasonic conditions, 1.5g LiBOB, 1.24g SN (the molar ratio of SN to LiBOB is 2:1) and 0.8g nano-magnesium oxide (particle size is 10nm) were sequentially added to 150g ethanol to obtain a uniform white Suspension; Then, add 8 g of molecular weight 8×10 5 PVA (structural unit is vinyl alcohol, the molar ratio of this structural unit and lithium salt is 23.5: 1), after ultrasonic stirring for 8 hours, obtain the macromolecule suspension (viscosity is 9.5Pa. s); then, the resulting polymer suspension is evenly coated on the flat glass surface by spin coating; finally, the glass coated with the polymer suspension is dried at 50° C. for 10 hours in a nitrogen atmosphere Polymer electrolyte membrane No. 1 was obtained.
Embodiment 2
[0029] Embodiment 2, preparation No. 2 polymer electrolyte
[0030] Under the condition of stirring, 2g LiBOB and 3g PC (the molar ratio of PC and LiBOB is 3.3: 1) are successively added in 200g N-methylpyrrolidone, obtain uniform transparent solution; Then, add 12g molecular weight to transparent solution 1×10 5PVdF (the structural unit is 1,1-difluoroethylene, the molar ratio of the structural unit to the lithium salt is 18:1), after stirring for 8 hours, a uniform milky white polymer solution (viscosity is 5.2Pa·s); Next, apply the obtained polymer solution on a flat glass surface by doctor blade coating; finally, dry the glass coated with the polymer solution at 80°C for 15 hours under a vacuum condition of -90KPa The No. 2 polymer electrolyte was obtained.
Embodiment 3
[0031] Embodiment 3, preparation No. 3 polymer electrolyte
[0032] Under the condition of ultrasonic stirring, 1.8g LiBOB and 2g nano-alumina (particle diameter is 100 nanometers) are added successively to 120g by acetone, N,N-dimethylformamide and petroleum ether (volume ratio is 1: 1: 2) In the mixed solution of composition, obtain uniform suspension; Then, add 14g molecular weight to this suspension and be 1 * 10 6 PMMA (structural unit is methyl methacrylate, the molar ratio of this structural unit and lithium salt is 15: 1), after stirring for 15 hours, obtains uniform milky white polymer suspension (viscosity is 12.2Pa·s); Then, the obtained polymer solution was coated on a flat polytetrafluoroethylene surface by the doctor blade coating method; finally, the polytetrafluoroethylene plate coated with the polymer suspension was dried in a carbon dioxide atmosphere at 60°C for 13 After 1 hour, No. 3 polymer electrolyte was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com