Co-deposition electroplating method with cyanogen-free Au-Sn alloy electrolyte
An alloy electroplating solution and co-deposition technology are applied in the field of cyanide-free Au-Sn alloy electroplating solution, which can solve the problems of slow plating speed and unstable plating solution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
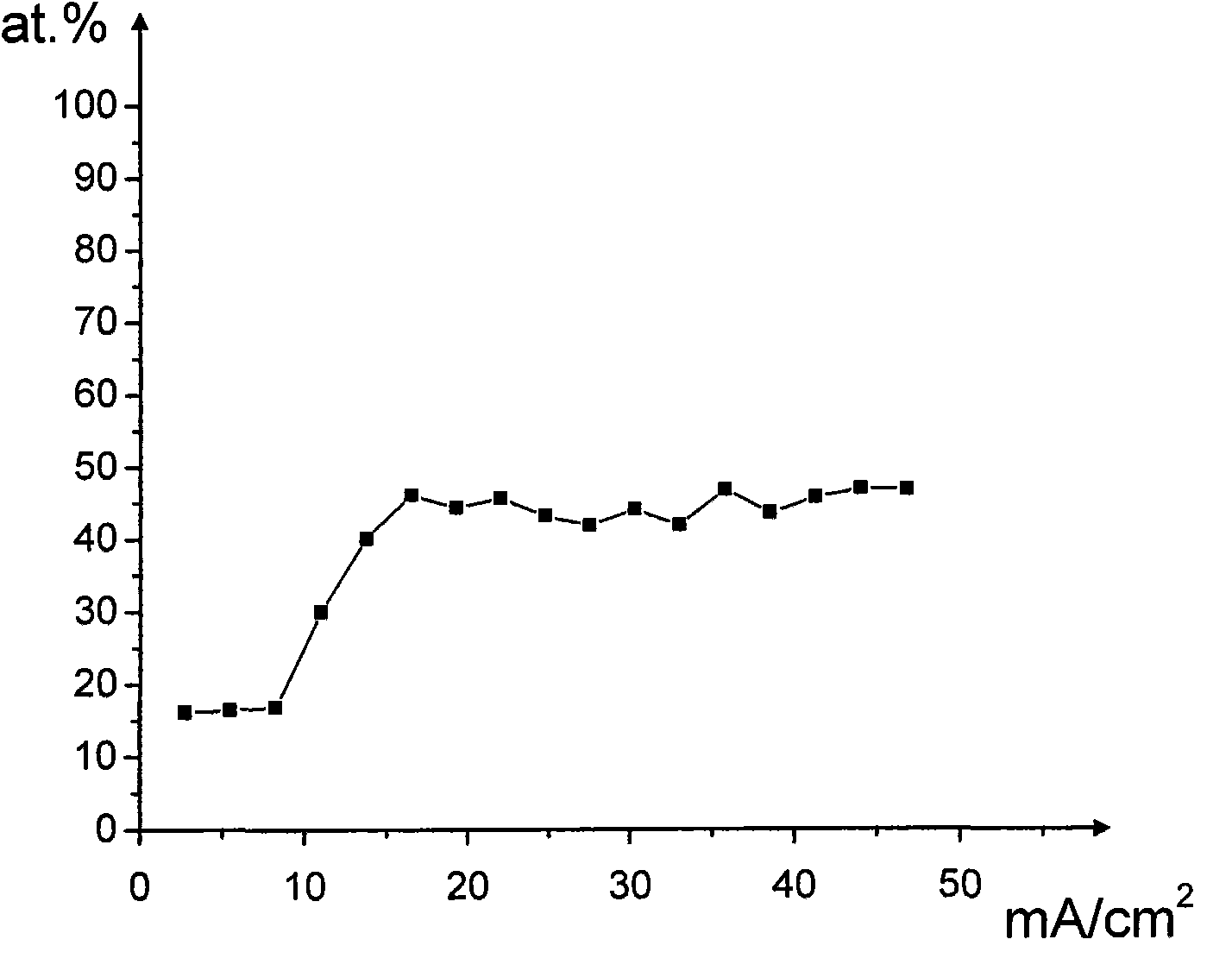
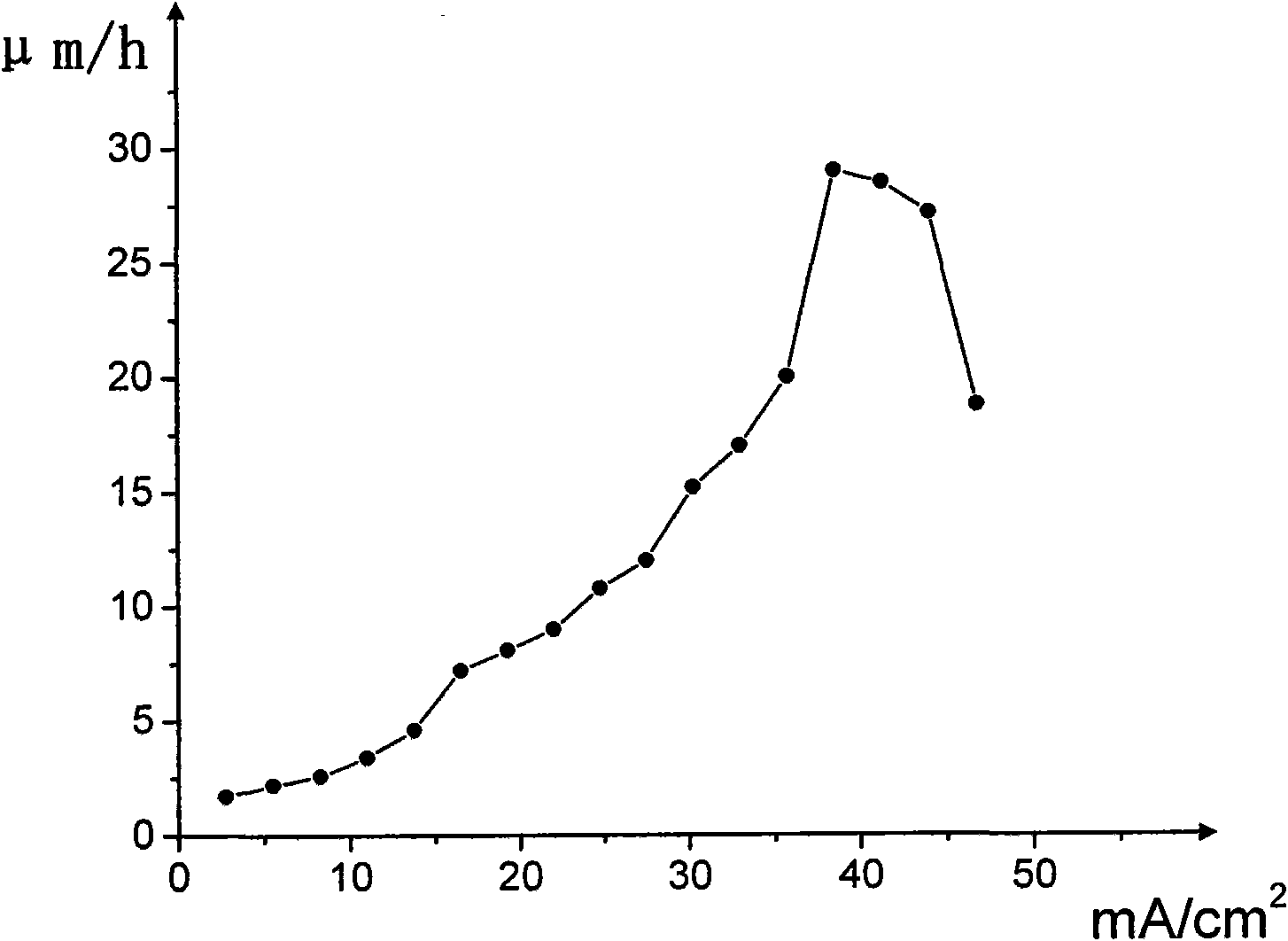
[0060] Embodiment 1: Au-30at.% Sn eutectic coating is electroplated with positive variable amplitude pulse current and the composition of the plating solution of the present invention
[0061] (1) Preparation of Au-Sn alloy electroplating solution
[0062] 1. The composition and content of the plating solution are as follows:
[0063] Potassium chloroaurate (KAuCl 4 ) 10g / L (5.8g / L as Au)
[0064] Stannous chloride (SnCl 2 ) 5g / L (3.13g / L based on Sn)
[0065] Sodium sulfite (Na 2 SO 3 ) 60g / L
[0066] Ethylenediaminetetraacetic acid (EDTA) 5g / L
[0067] Potassium pyrophosphate 40g / L
[0068] L-Ascorbic Acid 15g / L
[0069] Catechol 5g / L
[0070] Nickel Chloride (NiCl 2 ) 1g / L
[0071] 2. The preparation steps are as follows:
[0072] 1) Take 3 / 4 of the volume of deionized water to prepare the plating solution in a beaker (the total volume of the plating solution is 40mL), and add potassium pyrophosphate, stannous chloride, catechol, L-ascorbic acid, chlorine Nick...
Embodiment 2
[0094] Embodiment 2: Au-30at.% Sn eutectic coating is electroplated with a positive variable amplitude pulse current and the plating solution of the present invention
[0095] Bath composition and parameters:
[0096] Sodium gold sulfite (Na 3 Au(SO 3 ) 2 ) 15g / L (7g / L as Au)
[0097] Stannous Sulfate (SnSO 4 ) 9g / L (5g / L based on Sn)
[0098] Sodium sulfite (Na 2 SO 3 ) 80g / L
[0099] Ethylenediaminetetraacetic acid (EDTA) 15g / L
[0100] Potassium pyrophosphate (K 4 P 2 o 7 ) 60g / L
[0101] L-Ascorbic acid 30g / L
[0102] Catechol 2g / L
[0103] Nickel Chloride (NiCl 2 ) 0.5g / L
[0104] pH 8
[0105] The preparation steps of the plating solution are the same as in Example 1. The Au-Sn electroplating solution can be stored at room temperature for about 2 months, and the plating solution does not decompose when heated to 50° C., and has good stability.
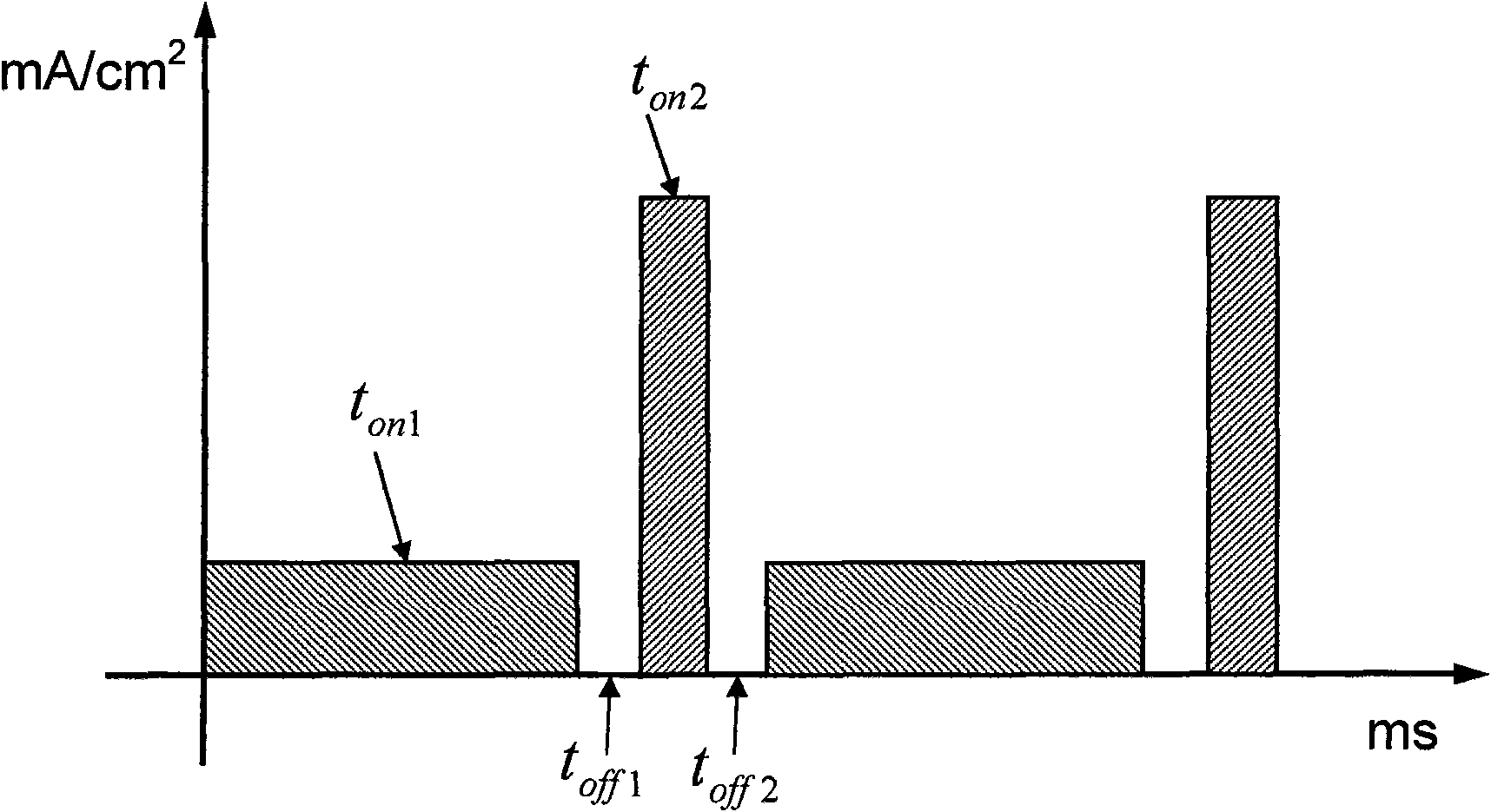
[0106] use as Figure 5 The positive-going periodic pulse shown (period 10ms, duty ratio 2:8, on-time 2ms) w...
Embodiment 3
[0113] Embodiment 3: Au-Sn alloy coatings (Au-30at.%Sn, Au-35at.%Sn) with different tin contents are prepared by electroplating with positive variable amplitude pulse current and plating solution of the present invention
[0114] Bath composition and parameters:
[0115] Ammonium Chloraurate (NH 4 AuCl 4 ) 7.2g / L (4g / L as Au)
[0116] Stannous methanesulfonate 7.76g / L (3g / L based on Sn)
[0117] Sodium sulfite (Na 2 SO 3 ) 40g / L
[0118] Ethylenediaminetetraacetic acid (EDTA) 3g / L
[0119] Potassium pyrophosphate 30g / L
[0120] L-Ascorbic acid 10g / L
[0121] Catechol 8g / L
[0122] Nickel Chloride (NiCl 2 ) 3g / L
[0123] pH 9
[0124] The preparation steps of the plating solution are the same as in Example 1. The Au-Sn plating solution can be placed at room temperature for about 2 months, and the plating solution does not decompose when heated to 50° C., and has good stability.
[0125] Using the same commutation cycle pulse and measurement steps as in Example 1, d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Peak current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com