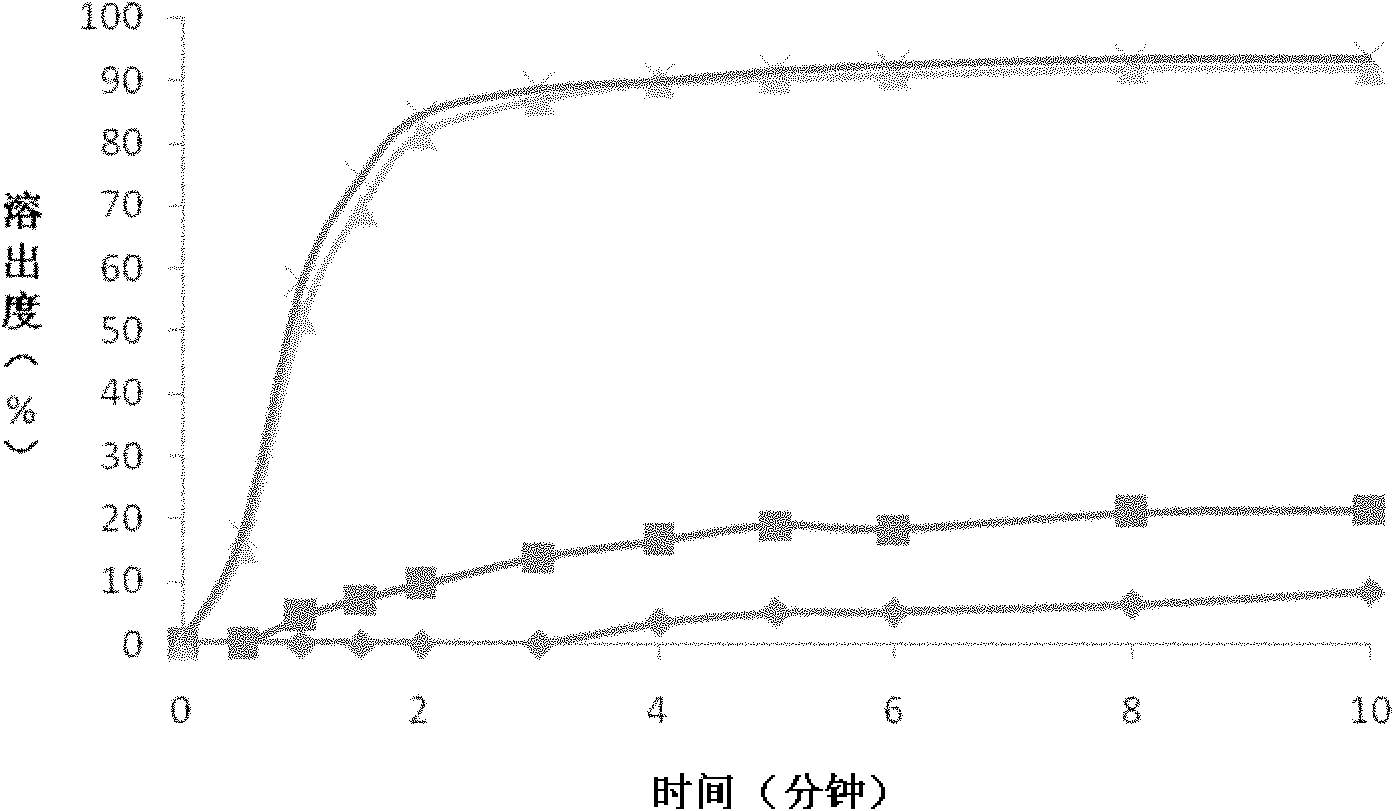
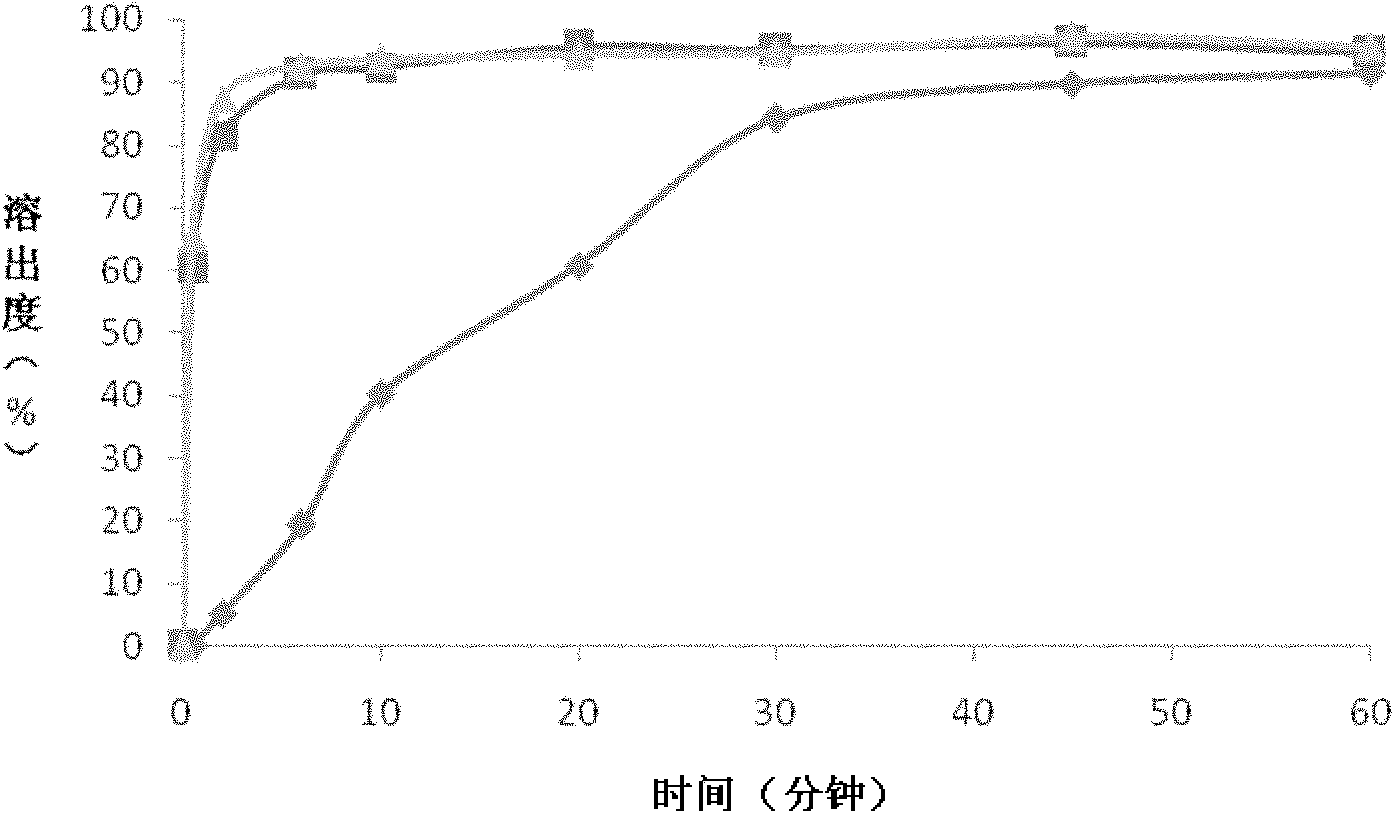
Nimodipine oral fast dissolving tablets and preparation method thereof
A nimodipine buccal, nimodipine technology, applied in nimodipine buccal instant tablets and its preparation field, can solve the problems of unacceptable and inconvenient use for patients, achieve simple preparation process, easy industrial production, avoid gastric Effects of intestinal first-pass
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Preparation of nimodipine solid dispersion: take nimodipine and polyvinylpyrrolidone in a ratio of 1:3, dissolve together in absolute ethanol, and ultrasonically mix the mixed solution for 15 minutes to form a uniform transparent liquid, then this The mixed solution is placed in a spray dryer for spray drying, and the dry powder is collected to obtain a nimodipine solid dispersion. The parameters of the spray dryer are set as: feed flow rate 20ml / min; spray pressure 7bar; atomizer rotation speed 50Hz; inlet air temperature 55-65°C; outlet air temperature 40-50°C.
[0030] (2) Preparation of nimodipine oral instant tablet: take 80 grams of nimodipine solid dispersion, 40 grams of microcrystalline cellulose, 24 grams of sodium bicarbonate, 30 grams of cross-linked polyvinylpyrrolidone, 10 grams of citric acid, cyclamate 10 grams, 5 grams of micropowder silica gel and 1 gram of magnesium stearate, after sieving and mixing evenly, use the powder direct compression metho...
Embodiment 2
[0034] (1) Preparation of nimodipine solid dispersion: take nimodipine and polyvinylpyrrolidone in a ratio of 1:6, dissolve together in absolute ethanol, and ultrasonically mix the mixed solution for 15 minutes to form a uniform transparent liquid, and then this The mixed solution is placed in a spray dryer for spray drying, and the dry powder is collected to obtain a nimodipine solid dispersion. The parameters of the spray dryer are set as: feed flow rate 20ml / min; spray pressure 7bar; atomizer rotation speed 50Hz; inlet air temperature 55-65°C; outlet air temperature 40-50°C.
[0035] (2) Preparation of nimodipine oral instant tablet: take 140 grams of nimodipine solid dispersion, 84 grams of microcrystalline cellulose, 40 grams of sodium bicarbonate, 84 grams of cross-linked polyvinylpyrrolidone, 20 grams of citric acid, cyclamate 20 grams, 10 grams of micro-powdered silica gel and 2 grams of magnesium stearate, sieved and mixed evenly, and compressed by direct powder compr...
Embodiment 3
[0039] (1) Preparation of nimodipine solid dispersion: take nimodipine and polyethylene glycol in a ratio of 1:3, dissolve them in absolute ethanol together, and ultrasonically mix the mixed solution for 15 minutes to form a uniform transparent liquid, and then mix The mixed solution is placed in a spray dryer for spray drying, and the dry powder is collected to obtain a nimodipine solid dispersion. The parameters of the spray dryer are set as: feed flow rate 20ml / min; spray pressure 7bar; atomizer rotation speed 50Hz; inlet air temperature 55-65°C; outlet air temperature 40-50°C.
[0040] (2) Preparation of nimodipine oral instant tablet: take 80 grams of nimodipine solid dispersion, 32 grams of microcrystalline cellulose, 22 grams of sodium bicarbonate, 40 grams of cross-linked polyvinylpyrrolidone, 10 grams of citric acid, cyclamate 10 grams, 5 grams of micropowder silica gel and 1 gram of magnesium stearate, after sieving and mixing evenly, use the powder direct compressio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com