Catalyst for catalytically hydrolyzing sodium borohydride to prepare hydrogen and preparation method thereof
A technology for catalyzing sodium borohydride and sodium borohydride, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. Introduce problems such as surfactant residues and catalytic effects, to achieve good surface wettability, reduce catalyst costs, and low prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The present invention also designs a catalyst preparation method for catalyzing the hydrolysis of sodium borohydride to produce hydrogen (hereinafter referred to as the preparation method). The preparation method adopts an in-situ reduction method. The specific implementation process steps are as follows:
[0020] (1) Preparation of silica carrier: first make 5-25ml of 28wt% ammonia water and 175-195ml of ethanol into a mixed solution, then add 2-20ml of ethyl orthosilicate to the mixed solution and stir to react After 6-20 hours, a silica carrier particle suspension is prepared; then the silica carrier particles in the suspension are precipitated on the bottom of the container by centrifugal separation, the upper liquid is removed, distilled water is added again, and the silica carrier particles deposited at the bottom are ultrasonically dispersed , The centrifugal separation is repeated 2-4 times, until the pH of the suspension is 7-8;
[0021] (2) Coated nickel or nickel ...
Embodiment 1
[0035] Preparation of metal-silica composite catalyst particles.
[0036] (1) Take 5ml of 28wt% ammonia and 195ml of ethanol, add 5ml of ethyl orthosilicate, stir and react for 10 hours to obtain a silica particle suspension; the obtained silica particles are separated by centrifugal separation to precipitate the silica particles At the bottom of the container, remove the upper liquid, re-add distilled water, ultrasonically disperse the particles at the bottom, repeat 3 times, until the pH of the suspension is 7-8.
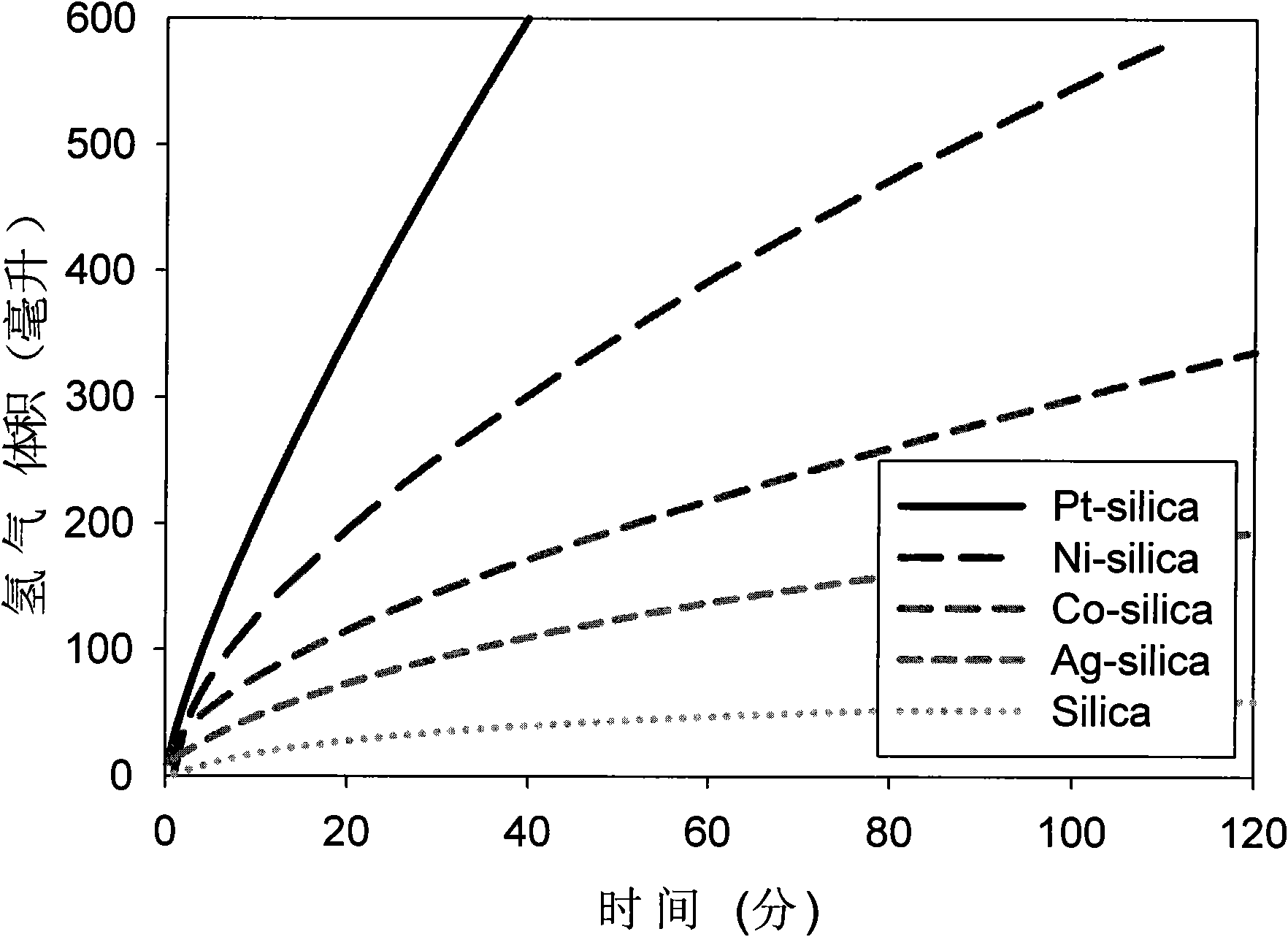
[0037] (2) Take 100 ml of the silicon oxide suspension solution, add 10 ml of the precursor of the aqueous solution of the metal salt (including platinum chloric acid, silver nitrate, nickel chloride or cobalt chloride, etc.) of 10 mmol / L, and stir and mix well. After adding excess reducing agent sodium borohydride to react, a composite catalyst suspension solution of each metal (platinum, silver, nickel, cobalt, etc.) nanoparticles coated with silicon oxide particles ...
Embodiment 2
[0040] Preparation of nickel boride-silicon oxide composite catalyst suspension.
[0041] (1) Mix 10 ml of 28wt% ammonia water with 180 ml of ethanol, add 10 ml of ethyl orthosilicate, stir and react for 12 hours to obtain a silica particle suspension; the obtained silica particle suspension is centrifuged to separate the silica particles Precipitate at the bottom of the container, remove the upper layer of liquid, re-add distilled water, ultrasonically disperse the particles precipitated at the bottom, repeat 4 times until the pH of the suspension is 7.
[0042] (2) Add 30 ml of 10 millimoles per liter of nickel chloride solution to 100 ml of silica suspension solution, stir and mix uniformly, add excess reducing agent sodium borohydride, and obtain a composite catalyst coated with NiB nanoparticles after the reaction suspension.
[0043] Take 1 ml of the above composite catalyst suspension and add it to 50 ml of an aqueous solution containing 75 millimoles of sodium borohydride, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com