2'-Aminochalcone azole compounds and their pyrazoline and cyclopropyl azole derivatives, preparation method and use
A technology of compound and application, applied in the fields of 2′-aminochalcone azole compounds and their pyrazoline and cyclopropylazole derivatives, antimicrobial and antitumor activities, capable of solving α-azolyl chalcone Less ketones and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
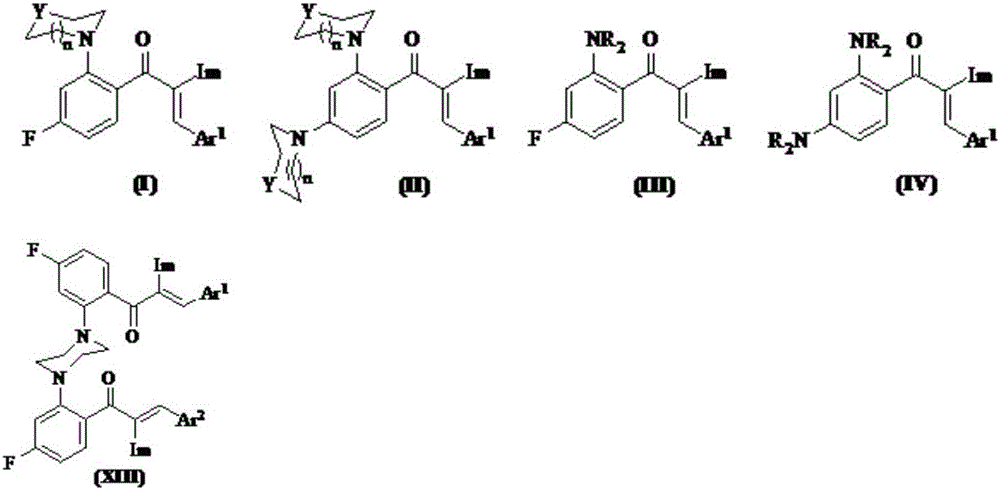
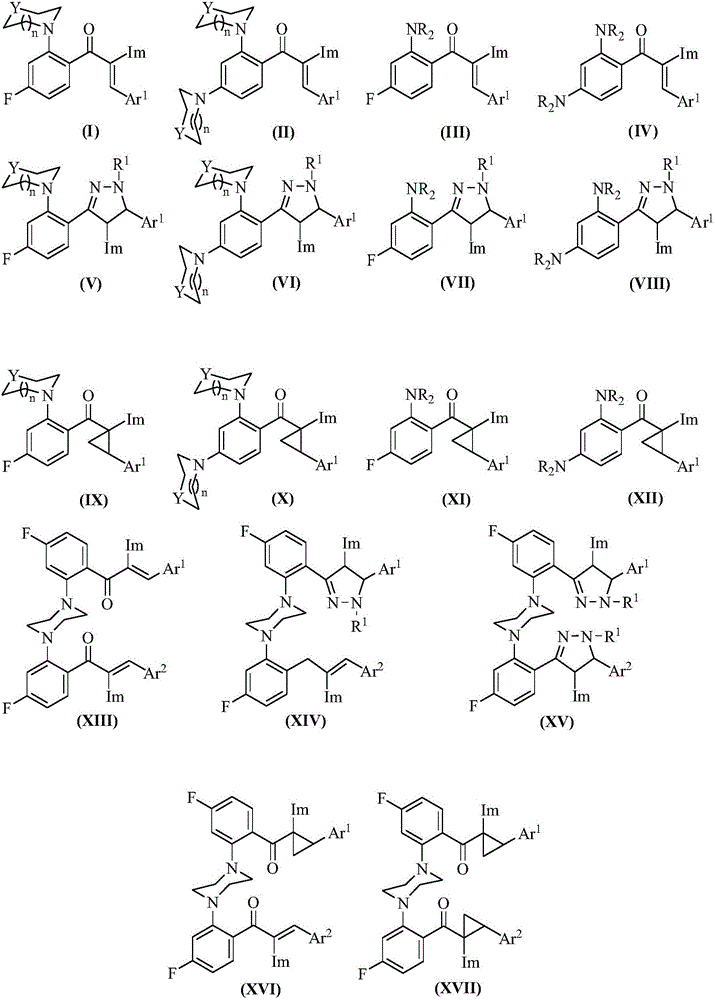
Image
Examples
Embodiment 1
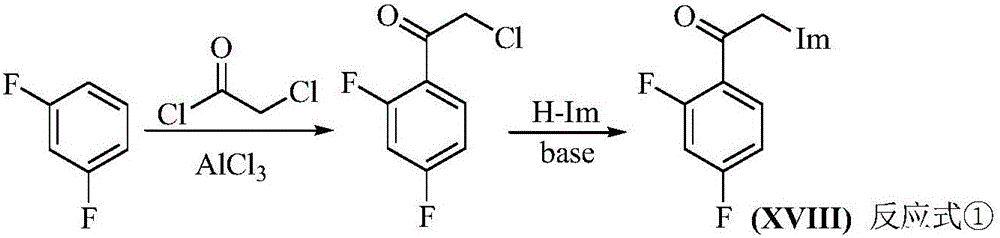
[0067] Example 1: Preparation of 2′,4′-difluoro-2-(1H-1,2,4-triazol-1-yl)acetophenone (referred to as compound 1)
[0068]
[0069] Add triazole (6.9g, 0.1mol) and potassium carbonate (13.8g, 0.1mol) into a 250mL round bottom flask, take an appropriate amount of acetonitrile as a solvent, and add 2′,4′-difluoro-2-chlorophenethyl Ketone (19.0 g, 0.1 mol). After the reaction, the solvent was evaporated to dryness, and an equal volume of water and chloroform was added to extract three times. The organic phase was washed with saturated ammonium chloride solution and distilled water, and dried over anhydrous sodium sulfate. After rotary evaporation and recrystallization, 14.5 g of light yellow solid was obtained, yield: 65%, melting point: 108-110°C (commercial product 107-111°C).
Embodiment 2
[0070] Example 2: (Z)-1-(4-fluoro-2-(piperidin-1-yl)phenyl)-3-phenyl-2-(1H-1,2,4-triazole-1- Base) prop-2-en-1-one (referred to as compound 2) and (Z)-1-(2,4-bis(piperidin-1-yl)phenyl)-3-phenyl-2-(1H Preparation of -1,2,4-triazol-1-yl)prop-2-en-1-one (compound 3 for short)
[0071]
[0072] In a 100mL single-necked flask, add an appropriate amount of toluene as a solvent, and then add 2′,4′-difluoro-2-(1H-1,2,4-triazol-1-yl)acetophenone (2.23g, 10mmol) , benzaldehyde (1.06g, 10mmol) and piperidine (1.87g, 22mmol), then add 3 drops of glacial acetic acid as a catalyst, install a water separator, keep stirring at 130°C under reflux, and the reaction is complete after 6 hours. Toluene was distilled off under reduced pressure, and an equal volume of water and chloroform were added to extract three times. The organic phase was dried over anhydrous sodium sulfate and then concentrated. Column chromatography was performed using petroleum ether and ethyl acetate (7 / 1, V / V) as elue...
Embodiment 3
[0075] Example 3: (Z)-1-(4-fluoro-2-morpholinophenyl)-3-phenyl-2-(1H-1,2,4-triazol-1-yl)propane-2 -en-1-one (referred to as compound 4) and (Z)-1-(2,4-dimorpholinophenyl)-3-phenyl-2-(1H-1,2,4-triazole- Preparation of 1-yl) prop-2-en-1-one (compound 5 for short)
[0076]
[0077] According to the synthetic method of embodiment 2. Starting materials are 2',4'-difluoro-2-(1H-1,2,4-triazol-1-yl)acetophenone (2.23g, 10mmol), benzaldehyde (1.06g, 10mmol) and Morpholine (1.91g, 22mmol) gave compound 4 (yellow solid, 2.09g, yield: 56%, melting point: 123-124°C) and compound 5 (red syrup, 0.84g, yield: 19%).
[0078] Compound 4: 1 HNMR (300MHz, CDCl 3 )δ: 8.11(s, 1H, trizole5-H), 8.06(s, 1H, trizole3-H), 7.49~6.70(m, 9H, C=CH, PhH), 3.77~3.74(t, 4H, J= 4.2Hz, morpholino OCH 2 ),3.01~2.98(t,4H,J=4.5Hz,morpholinoNCH 2 )ppm; MS(m / z):379[M+H] + .
[0079] Compound 5: 1 HNMR (300MHz, CDCl 3 )δ: 8.11(s, 1H, trizole5-H), 8.06(s, 1H, trizole3-H), 7.50~6.42(m, 9H, C=CH, PhH), 3.89...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com