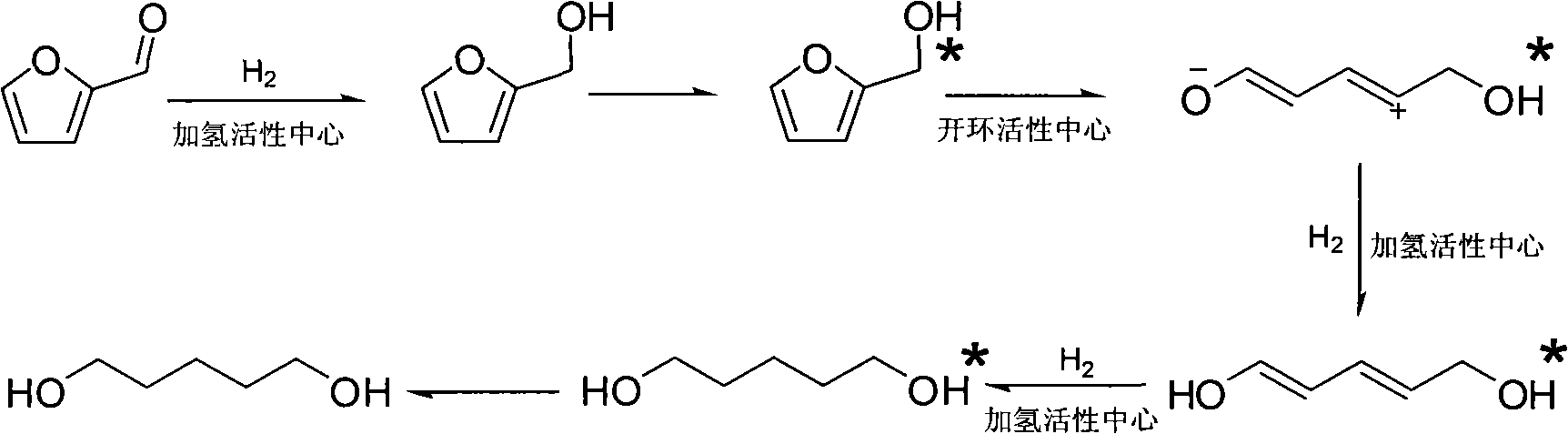
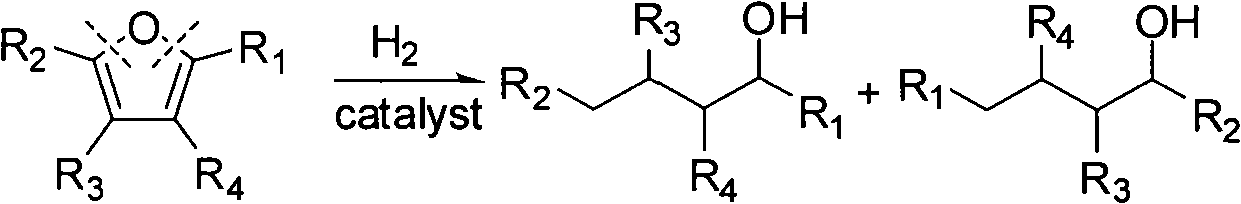
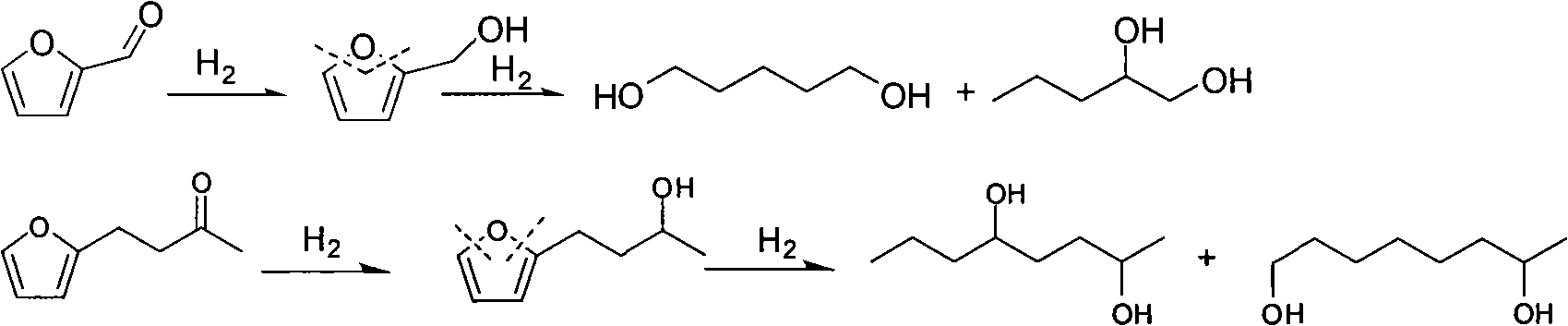
Catalyst used in ring-opening hydrogenation reaction of furan derivative
A technology of furan derivatives and hydrogenation catalysts, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, hydrogenation preparations, etc., can solve the problem of increasing reaction costs, harsh reaction conditions, and high reaction temperature problems, to achieve the effect of reducing reaction pressure and cost, mild reaction conditions and high catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-18
[0018] Preparation of catalyst by impregnation method: prepare a certain concentration of nitric acid I solution (I is Pt, Pd, Rh, Ru, Co or Ni) and nitric acid II solution (II is Re, Mo, Mn, Co, Ti, W, Cr, Fe, V or Ta), add in the carrier according to a certain stoichiometric ratio for dipping, dry overnight at 100°C, calcinate at 400°C for 4 hours, and reduce in hydrogen before use. The catalyst composition in the embodiment is shown in Table 1.
[0019] Table 1
[0020] Example
carrier
Composition (I)
Content (I)
Composition (II)
Content (II)
Example 1
Pt
0.5
ReO 2
20
Example 2
MgO
Pd
0.8
MnO 2
18
Example 3
Al 2 O 3
Rh
1.0
MoO 3
15
Example 4
BaO
Ru
1.5
Co 3 O 4
10
Example 5
Amorphous silicon aluminum
Co
2.0
TiO 2
8
Example 6
SiO 2
Ni
2.5
WO 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com