Preparation method of CuInS2 nanoparticles
A nanoparticle and solution technology, applied in the field of nanomaterials, achieves the effects of simple and unique method, simple synthesis steps, and avoiding physical methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
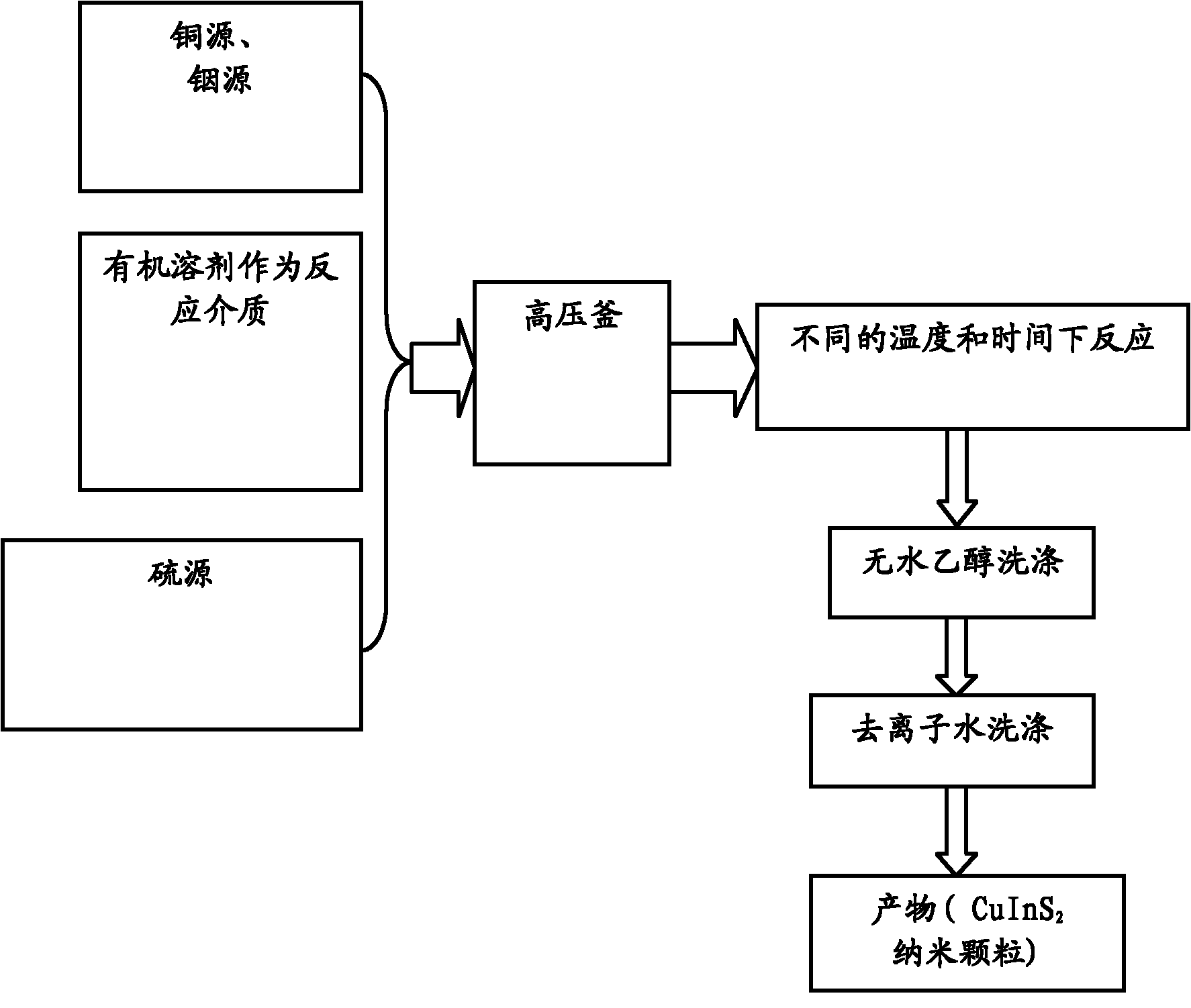
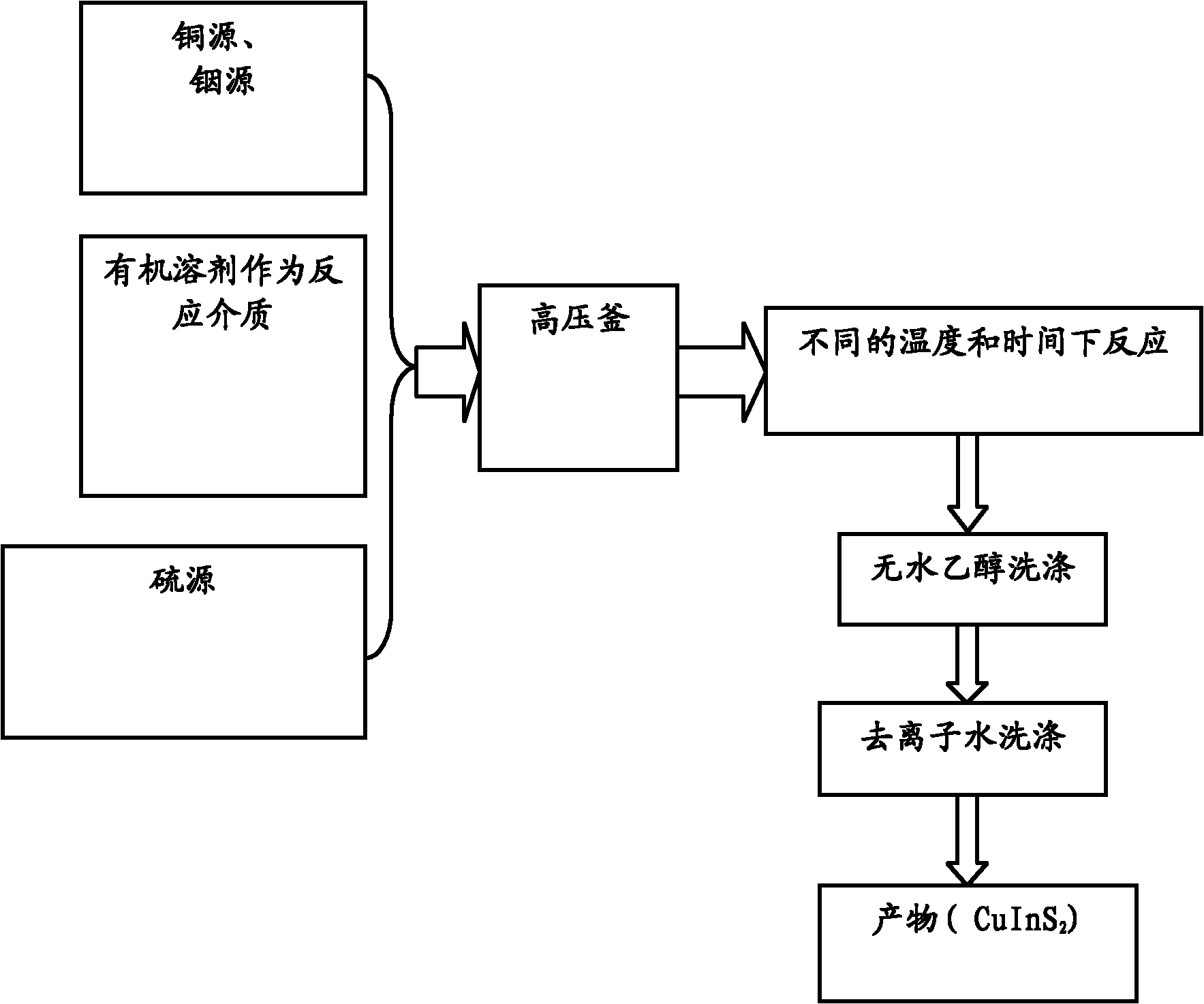
[0031] Weigh 1mmol copper chloride and dissolve it in 40ml DMF; then add 1mmol indium chloride to the above solution and stir to dissolve it; finally add 2.0mmol L-cysteine to the above mixture to make the copper source, The ratio of the amount of indium source to the sulfur source is 1:1:2, then add 1.5mol / L ammonia water dropwise under constant stirring, adjust the pH value of the solution to 8, and transfer it into a 50mL container after forming a clear solution. After sealing in a polytetrafluoroethylene-lined autoclave, keep the temperature in a drying oven at 200°C for 14 hours, and then cool naturally to room temperature. The obtained product was washed several times with absolute ethanol and deionized water to remove soluble matter, and finally dried in a vacuum oven at 60° C. for 6 hours to obtain the product.
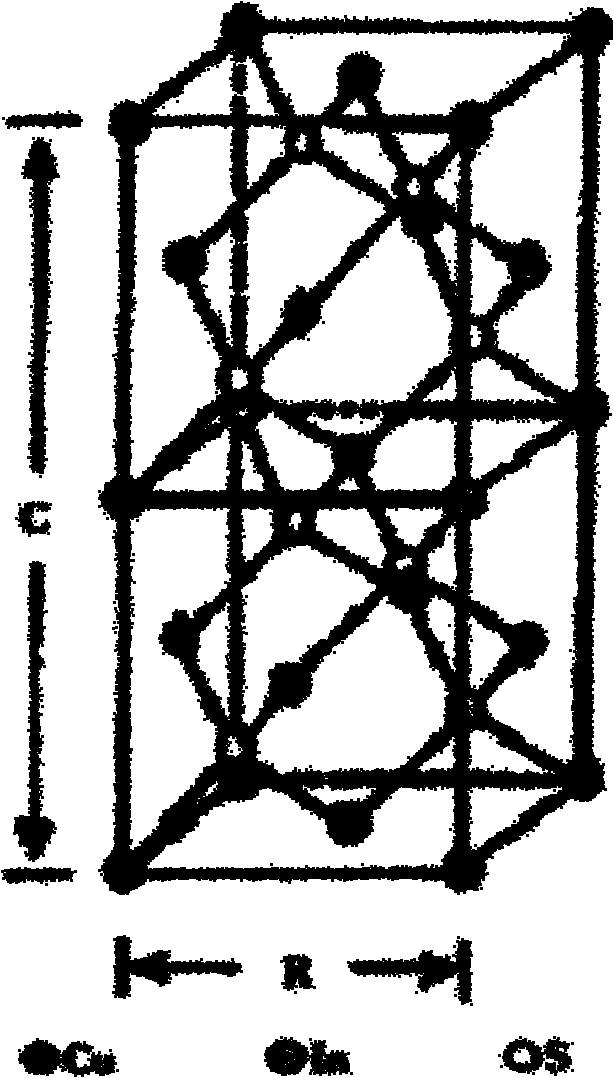
[0032] The chemical composition of the dried product was measured by X-ray photoelectron spectroscopy (XPS), and the molar ratio of Cu:In:S reached 1:1.03:1...
Embodiment 2
[0034] Same as Example 1, but the constant temperature reaction time is extended to 16h.
[0035] The products prepared under the same reaction conditions can be seen under the transmission electron microscope with more complete morphology, larger grain size and more obvious crystal phase. This is because the nuclei can grow larger with longer reaction times. At the same time, it shows that time has a great influence on the product morphology and crystal plane growth tendency. The chemical composition of the dried product was measured by X-ray photoelectron spectroscopy (XPS), and the molar ratio of Cu:In:S was 1.02:1:1.95.
Embodiment 3
[0037] Same as Example 1, but the constant temperature reaction temperature is reduced to 160°C.
[0038] The product prepared under the same conditions was detected and found that its morphology and composition did not change much, but the crystal phase changed greatly. The chemical composition of the dried product was measured by X-ray photoelectron spectroscopy (XPS), Cu:In : The molar ratio of S is 1.0:1.04:1.98, indicating that CuInS 2 The crystal phase of the crystal is very sensitive to the reaction temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com