Method for preparing structure controllable barium carbonate composite particles
A technology of composite particles and barium carbonate, which is applied in the treatment of dyed polymer organic compounds, fibrous fillers, etc., can solve the problems of high experimental cost, difficulty in realizing the structure and shape of barium carbonate particles, and precise regulation, so as to achieve a uniform reaction system , The preparation method is simple and easy to operate, and the effect of solvent pollution is small
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
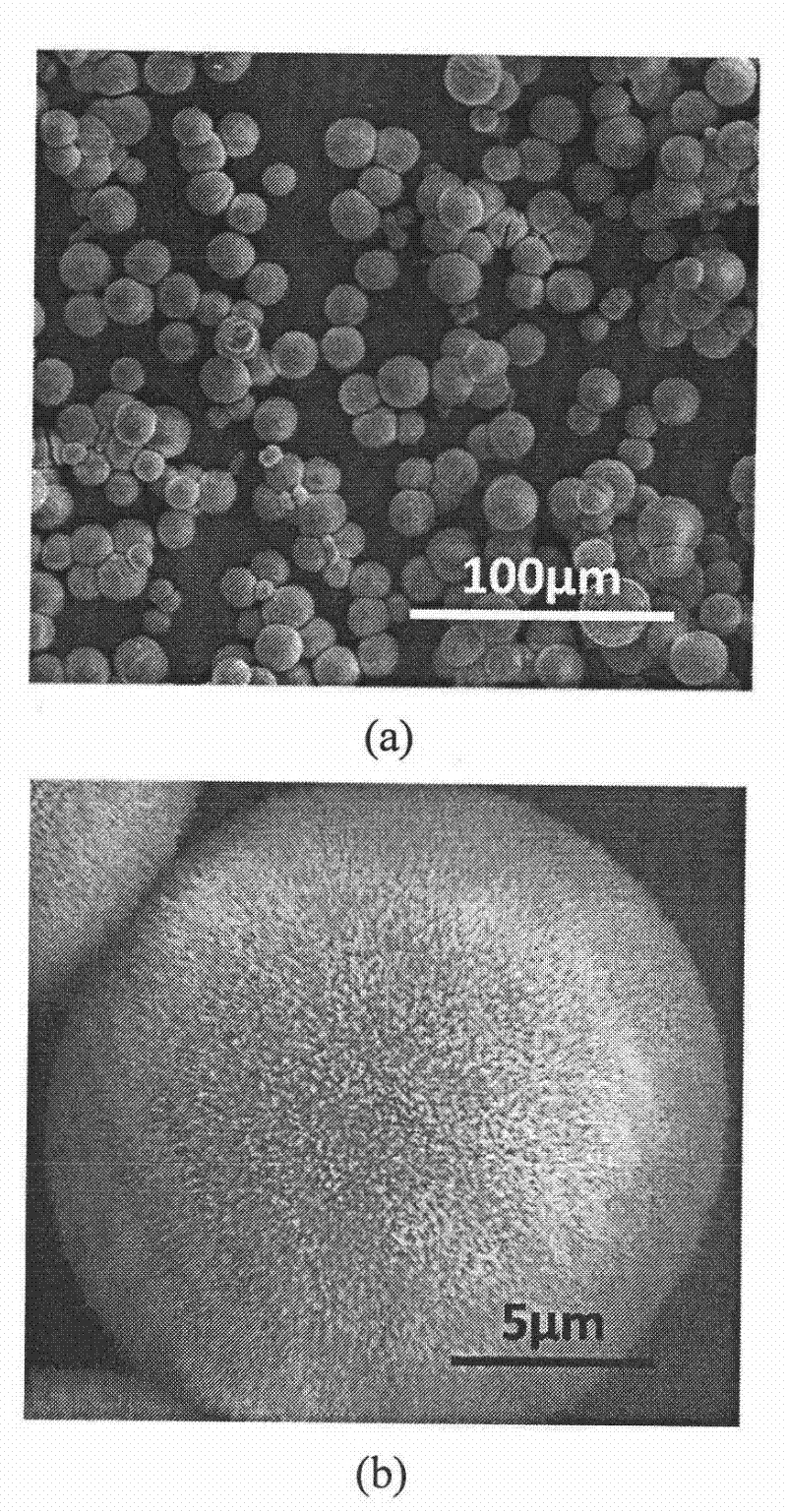
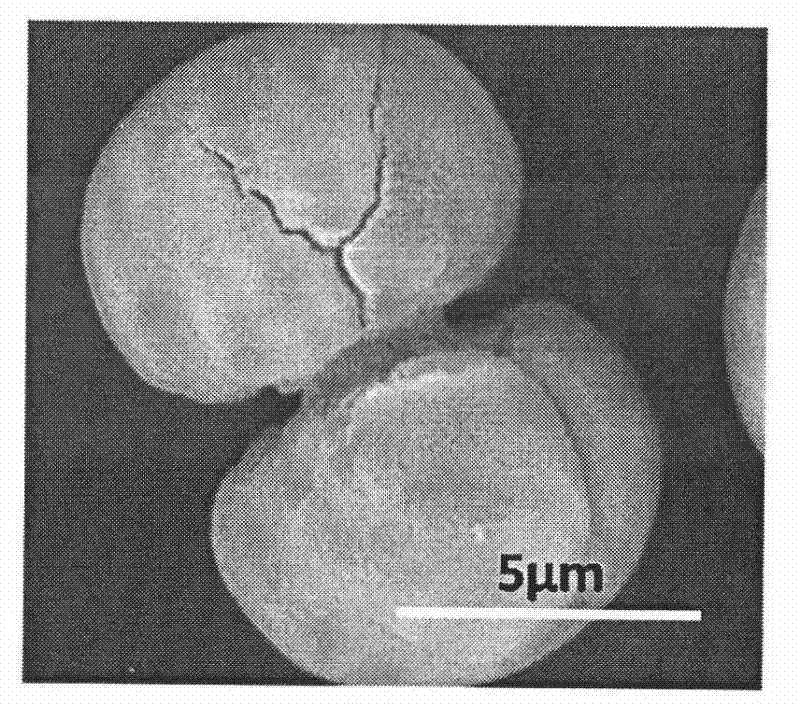
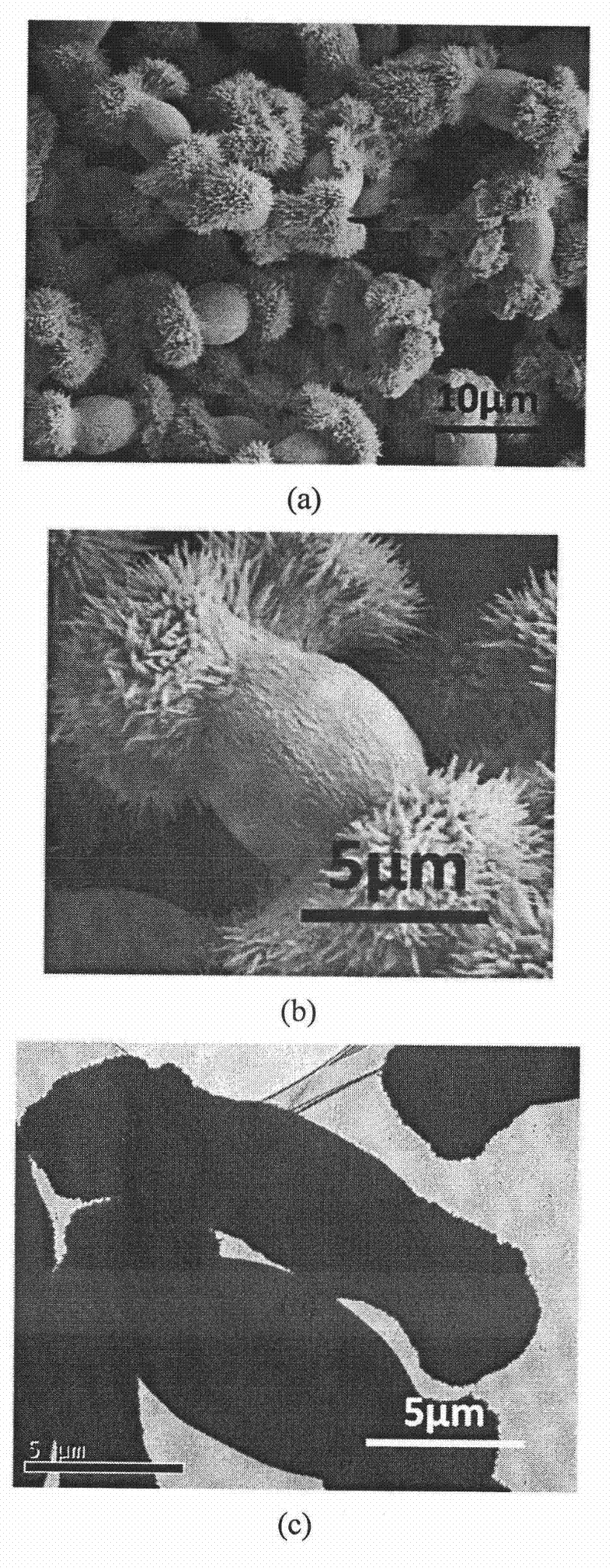
[0061] All glassware used in the experiments were pretreated by first sonicating in ethanol for 15 min, and then removed and rinsed with deionized water. Put it into a mixed solvent of concentrated nitric acid / hydrogen peroxide / water in a ratio of 1:1:1 and soak for 2 days, take it out, wash it with acetone, and dry it at room temperature. Prepare 100 milliliters of carboxymethyl cellulose (weight average molecular weight: 90,000) aqueous solution of 10 g / L, then add a certain amount of barium chloride to prepare a 0.01 mol / L barium chloride solution, and adjust the pH of the solution to 8. Cover the beaker containing the solution with aluminum foil, and prick 3 pinholes in the aluminum foil. Take 20 grams of ammonium carbonate, grind it to a powder with a fineness of 100 mesh, put it in a 50 ml beaker, cover the mouth of the beaker with a polyethylene film, and prick 10 holes with a needle. Then put it into a desiccator with a volume of about 10 liters together with the abov...
Embodiment 2
[0063] The glass instrument processing method is the same as in Example 1. Prepare 100 milliliters of carboxymethyl cellulose (weight-average molecular weight is 90,000) aqueous solution of 1 gram / liter, add a certain amount of barium chloride and be mixed with the barium chloride solution of 0.01 mol / liter, adjust the pH of solution to be 8.5, will contain The beaker with the solution was covered with aluminum foil, and 3 pinholes were pierced in the aluminum foil. Take 20 grams of ammonium carbonate, grind it to a powder with a fineness of 100 mesh, put it in a 50 ml beaker, cover the mouth of the beaker with a polyethylene film, and prick 10 holes with a needle. Then put it into a desiccator with a volume of about 10 liters together with the above-mentioned beaker containing the solution, seal it, and start the reaction at a temperature of 20±1°C. After reacting for 12 hours, the resulting suspension was centrifuged and washed alternately with water and ethanol three times...
Embodiment 3
[0065] The glass instrument processing method is the same as in Example 1. Prepare 100 milliliters of carboxymethylcellulose (weight-average molecular weight is 90,000) aqueous solution of 0.1 gram / liter, add a certain amount of barium chloride and be mixed with the barium chloride solution of 0.1 mol / liter, adjust the pH of solution to be 9, will contain The beaker with the solution was covered with aluminum foil, and 3 pinholes were pierced in the aluminum foil. Take 20 grams of ammonium carbonate, grind it to a powder with a fineness of 100 mesh, put it in a 50 ml beaker, cover the mouth of the beaker with a polyethylene film, and prick 10 holes with a needle. Then put it into a desiccator with a volume of about 10 liters together with the above-mentioned beaker containing the solution, seal it, and start the reaction at a temperature of 20±1°C. After reacting for 12 hours, the resulting suspension was centrifuged and washed alternately with water and ethanol three times e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com