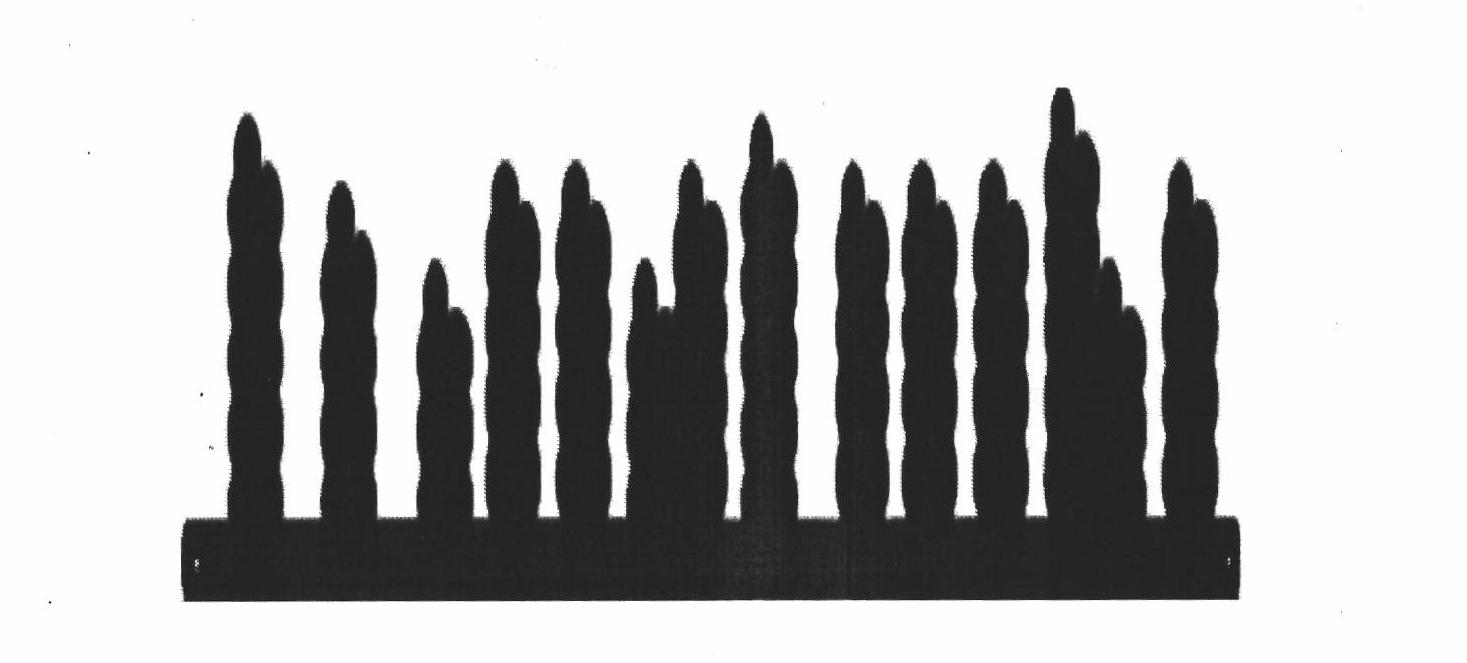
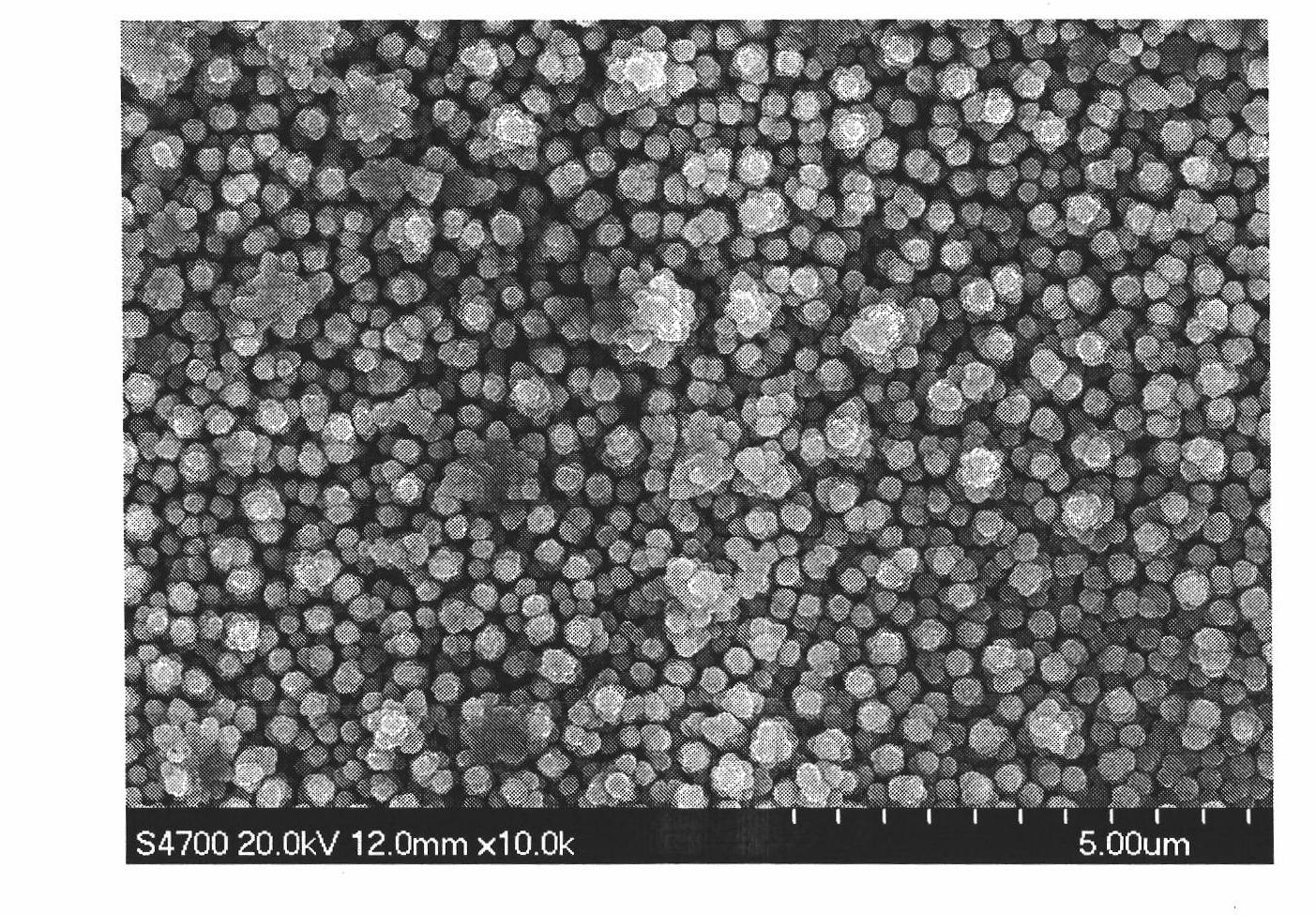
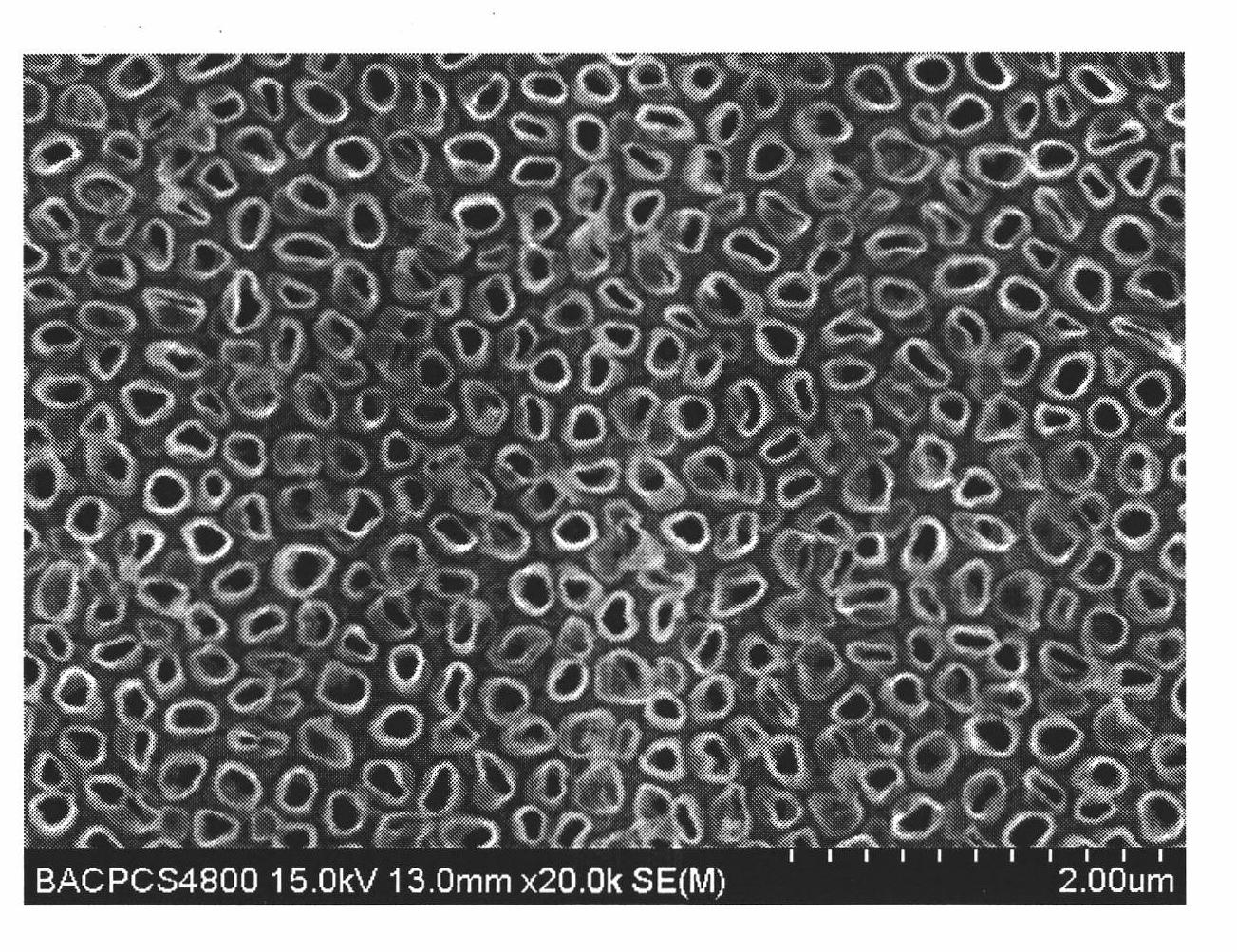
Method for preparing nickel and nickel alloy one-dimensional superstructure nanometer functional materials by adopting hydrogen separation template method
A nickel-based alloy and nano-functional technology, which is applied in the field of nickel and nickel-based alloy one-dimensional superstructure nano-functional materials prepared by hydrogen evolution template method, achieves the effect of simple and convenient operation, high efficiency and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The metal salt used in this example is: nickel chloride; the co-precipitated binary group is: stannous chloride; the additive is: potassium pyrophosphate; the pH value buffering agent is: glycine.
[0034] Step 1: Adhere 2×2cm copper foil on the copper plate, and perform pretreatment as the cathode of the electroplating process; that is, perform electropolishing treatment. The electropolishing solution is composed of concentrated phosphoric acid (98%) and deionized water, and the volume ratio of concentrated phosphoric acid: deionized water is 2:1. The copper plate is used as the cathode, and the copper foil to be treated is used as the anode for electropolishing, and the polishing potential is 2.5V. Take it out when the surface of the copper foil becomes mirror-like, rinse with deionized water and blow dry with nitrogen.
[0035] Step 2: NiCl 2 2H 2 O, SnCl 2 2H 2 O, K 4 P 2 o 7 , Glycine compound dissolved in deionized water, configured to contain 0.12mol / L Ni...
Embodiment 2
[0038] The main salt used in this example is: nickel chloride, nickel sulfate; the co-precipitated binary group is: phosphoric acid; the pH value buffer is: boric acid; the additive is: ammonia water.
[0039]Step 1: pre-treat the commercial AAO template, that is, ultrasonically clean the AAO template in a mixed solution of deionized water, ethanol, and acetone for 15 minutes.
[0040] Step 2: NiSO 4 ·6H 2 O, NiCl 2 ·6H 2 O, H 3 BO 3 、H 3 PO 4 , NH 3 ·H 2 The O compound is dissolved in deionized water and configured to contain 0.95mol / L NiSO 4 ·6H 2 O, 0.17mol / L NiCl 2 ·6H 2 O, 0.32mol / L H 3 BO 3 , 0.2-0.4mol / L H 3 PO 4 , NH 3 h 2 O electrolyte, adjust the pH value to 4.0, and keep the temperature at 30 degrees Celsius; use the cleaned AAO as the cathode, and the Pt sheet as the anode; at a constant current density of 1mA / cm 2 Under electroplating. The electroplating time is 120min.
[0041] Step 3: Put the product obtained in Step 2 in 3mol / L NaOH, etch f...
Embodiment 3
[0044] The main salt used in this example is: nickel chloride, nickel sulfate; the co-precipitation binary group is: sodium thiosulfate; the additive is: sulfosalicylic acid; the pH value buffer agent is: boric acid.
[0045] Step 1: Adhere 2×2cm copper foil on the copper plate, and perform pretreatment as the cathode of the electroplating process; that is, perform electropolishing treatment. The electropolishing solution is composed of concentrated phosphoric acid (98%) and deionized water, and the volume ratio of concentrated phosphoric acid: deionized water is 2:1. The copper plate is used as the cathode, and the copper foil to be treated is used as the anode for electropolishing, and the polishing potential is 2.5V. Take it out when the surface of the copper foil becomes mirror-like, rinse with deionized water and blow dry with nitrogen.
[0046] Step 2: NiSO 4 ·6H 2 O, NiCl 2 ·6H 2 O, H 3 BO 3 、Na 2 S 2 o 3 、C 7 h 6 o 6 The S compound is dissolved in deionize...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com