Salt-tolerant acrylic absorbent resin and method for preparing same
A technology of water-absorbent resin and acrylic acid, which is applied in the direction of chemical instruments and methods, and other chemical processes, can solve the problems of single type of chelating ions, single product function, and poor absorption capacity of chelating agents, so as to broaden adaptability and improve surface coagulation. Adhesive strength and the effect of improving the liquid absorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
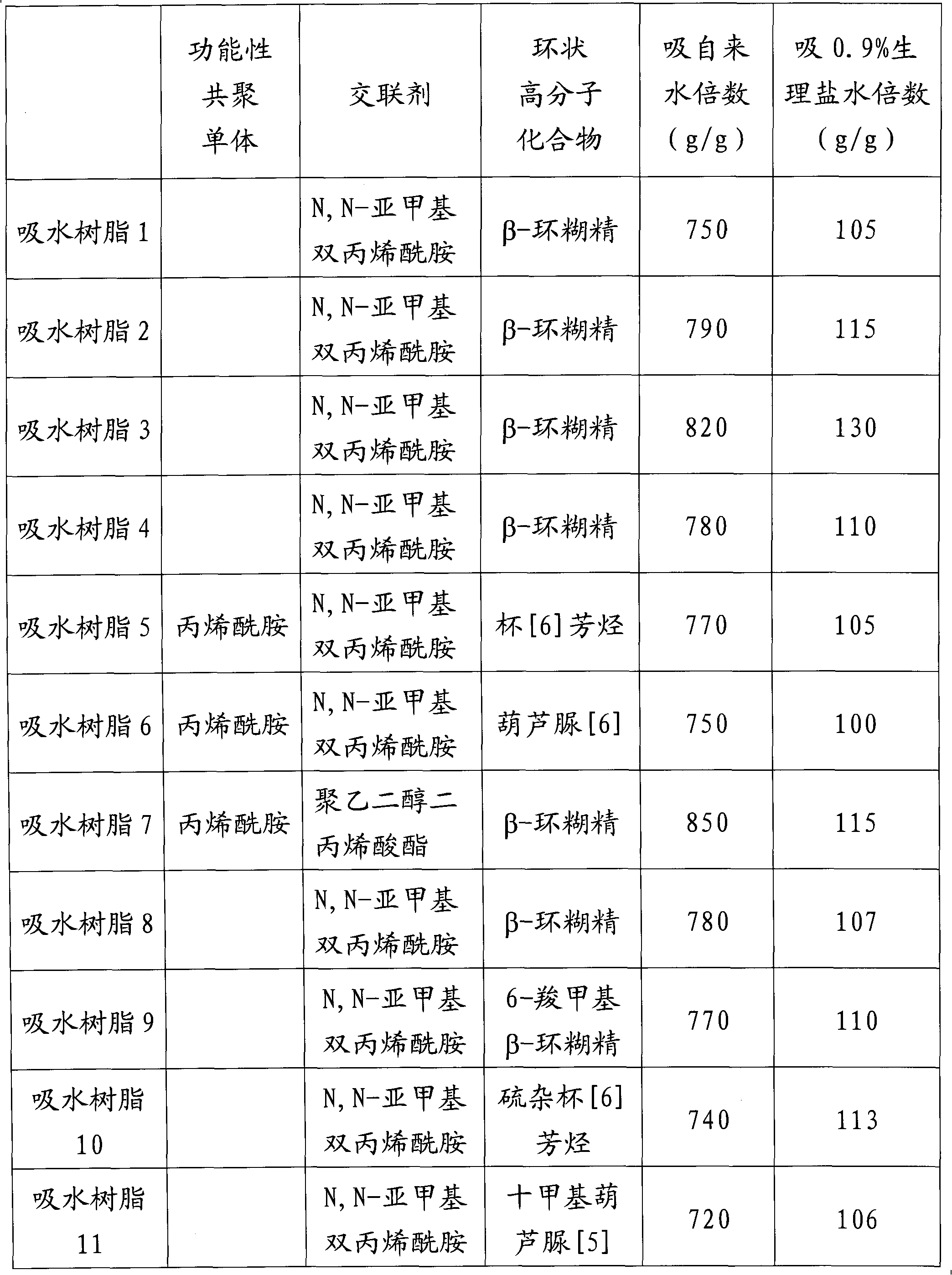
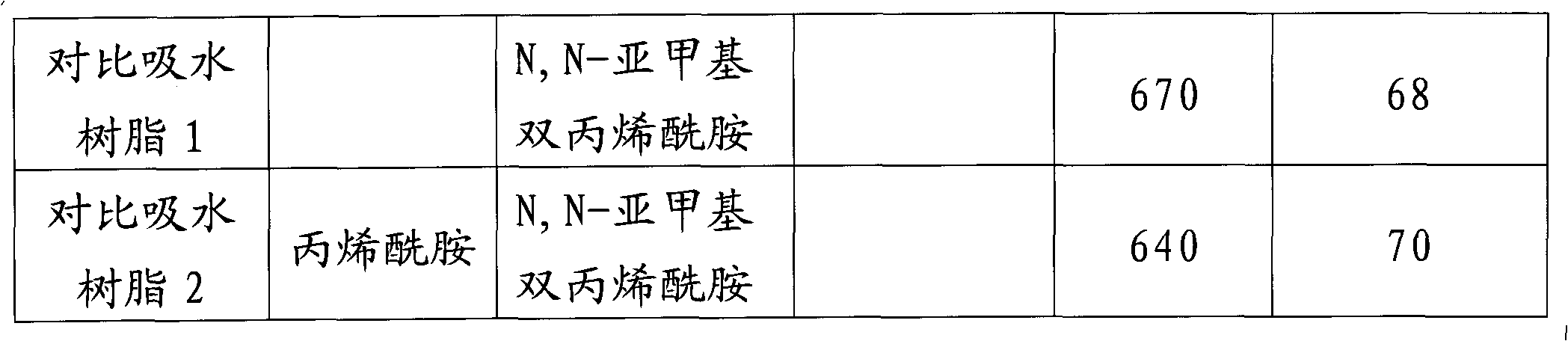
[0032]a) Add 2700g of high-purity acrylic acid and 800g of deionized water into a 10L reaction kettle with a stirrer and a jacket cooling device, and then slowly add 3100g of 32% by weight sodium hydroxide solution under stirring and cooling conditions Reactor for neutralization reaction.
[0033] b) The above sodium acrylate aqueous solution was fed into a jacketed double-arm kneader with a volume of 10 L and two sigma blades, and the air in the system was replaced with nitrogen while keeping the reaction liquid at 30°C. Then, while stirring the reaction liquid, 0.27 g of β-cyclodextrin, 0.54 g of N,N-methylenebisacrylamide, 8.1 g of potassium persulfate and 8.1 g of sodium bisulfite were added. Polymerization started quickly and the resulting hydrogel polymer was removed after 1 hour. The hydrogel polymer has been segmented into small pieces with a diameter of 3-10 mm.
[0034] c) The above-mentioned sodium acrylate hydrogel was quickly washed with 3 L of methanol, and fil...
Embodiment 2
[0038] a) Add 2700g of high-purity acrylic acid and 800g of deionized water into a 10L reaction kettle with a stirrer and a jacket cooling device, and then slowly add 3100g of 32% by weight sodium hydroxide solution under stirring and cooling conditions Reactor for neutralization reaction.
[0039] b) The above sodium acrylate aqueous solution was fed into a jacketed double-arm kneader with a volume of 10 L and two sigma blades, and the air in the system was replaced with nitrogen while keeping the reaction liquid at 30°C. Then, while stirring the reaction solution, 270 g of β-cyclodextrin, 0.54 g of N,N-methylenebisacrylamide, 8.1 g of potassium persulfate and 8.1 g of sodium bisulfite were added. Polymerization started quickly and the resulting hydrogel polymer was removed after 1 hour. The hydrogel polymer has been segmented into small pieces with a diameter of 3-10 mm.
[0040] c) The above-mentioned sodium acrylate hydrogel was quickly washed with 3 L of methanol, and f...
Embodiment 3
[0044] a) Add 2700g of high-purity acrylic acid and 800g of deionized water into a 10L reaction kettle with a stirrer and jacketed cooling device, and then slowly add 3752g of 32% by weight sodium hydroxide solution under stirring and cooling conditions Reactor for neutralization reaction.
[0045] b) The above sodium acrylate aqueous solution was fed into a jacketed double-arm kneader with a volume of 10 L and two sigma blades, and the air in the system was replaced with nitrogen while keeping the reaction liquid at 30°C. Then, while stirring the reaction solution, 27 g of β-cyclodextrin, 0.54 g of N,N-methylenebisacrylamide, 8.1 g of potassium persulfate and 8.1 g of sodium bisulfite were added. Polymerization started quickly and the resulting hydrogel polymer was removed after 1 hour. The hydrogel polymer has been segmented into small pieces with a diameter of 3-10 mm.
[0046] c) The above-mentioned sodium acrylate hydrogel was quickly washed with 3 L of methanol, and fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com